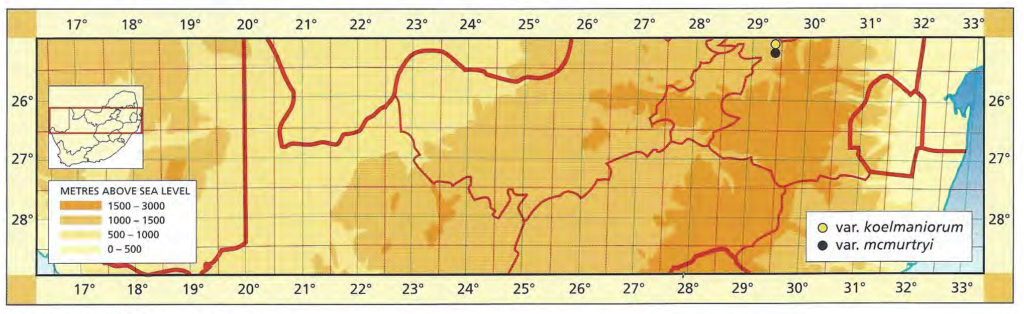
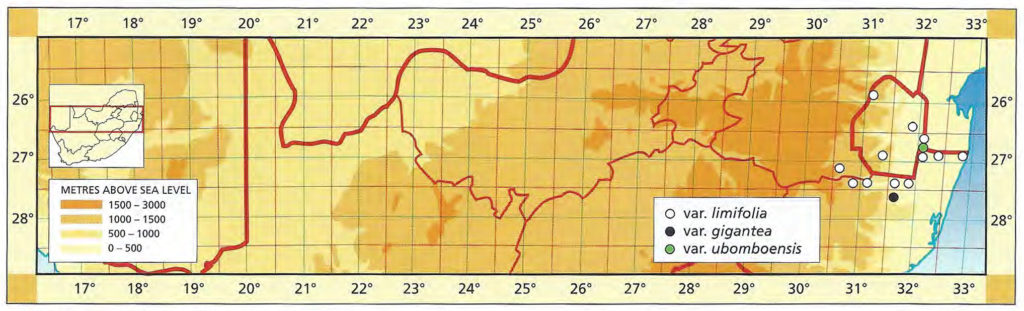
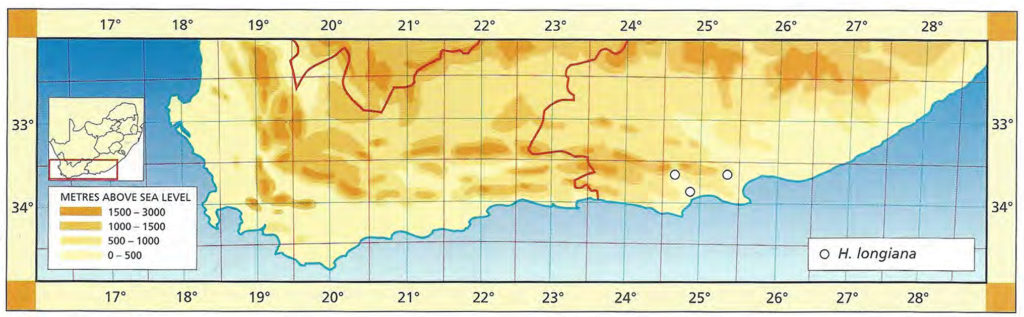
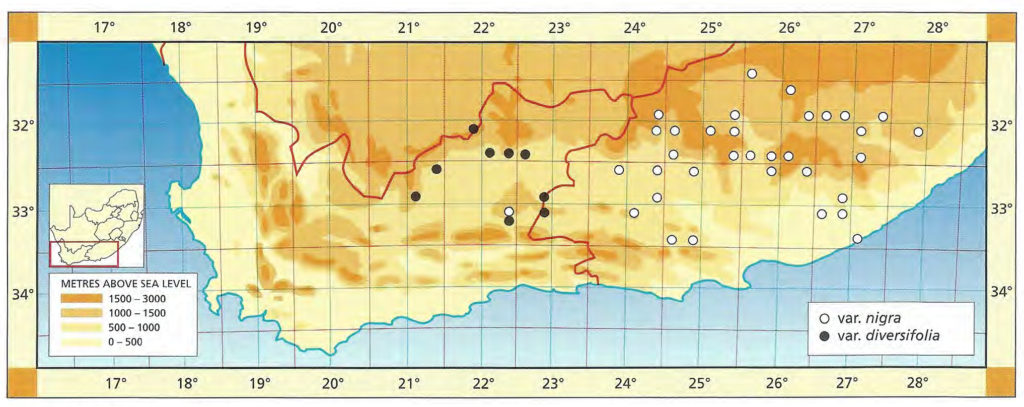
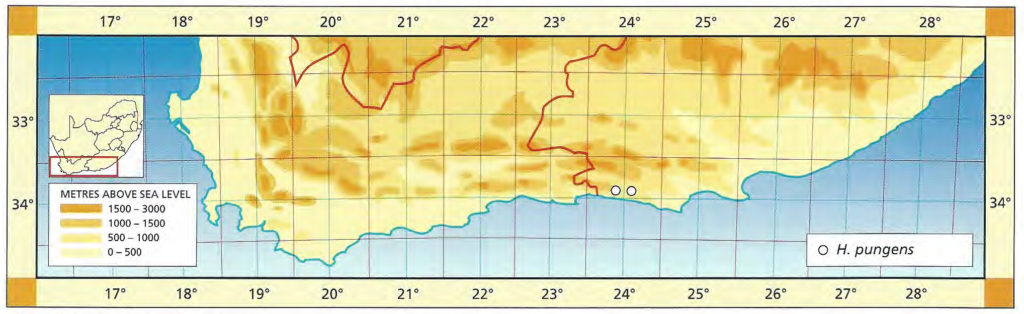
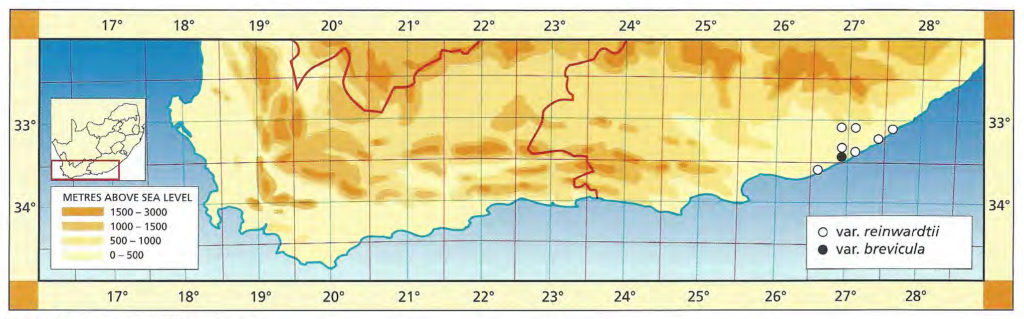
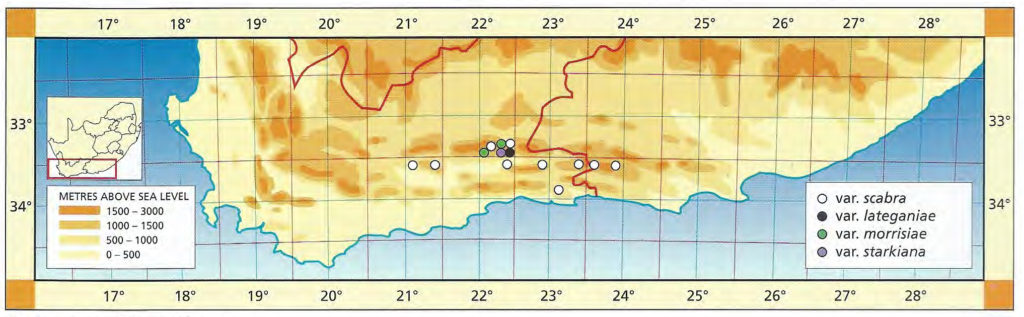
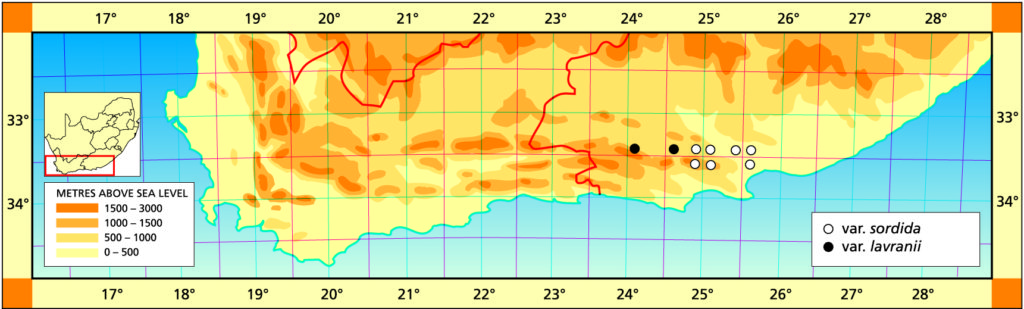
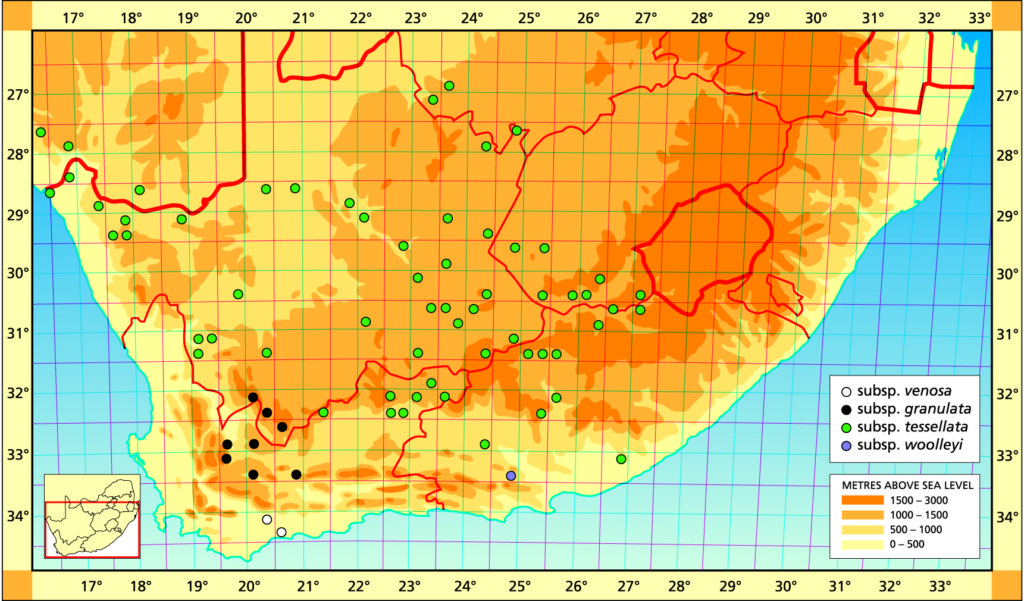
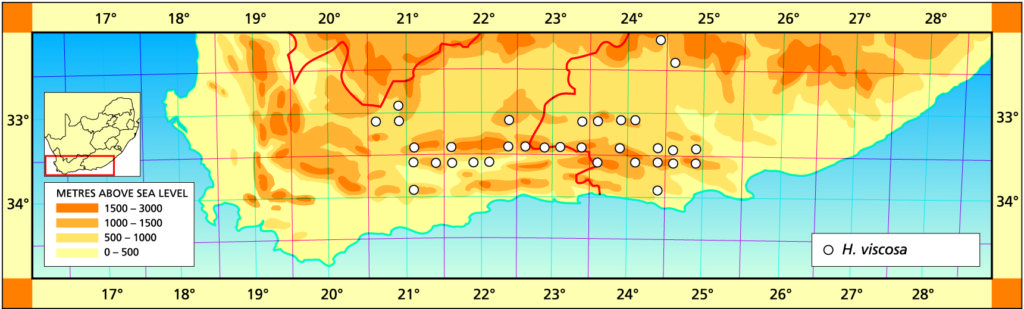
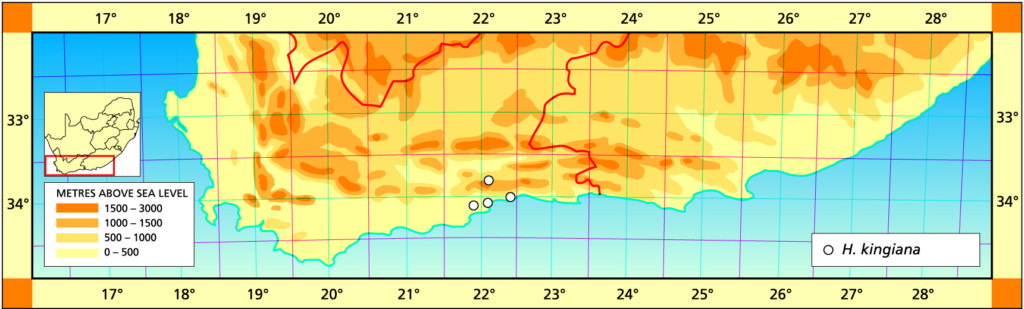
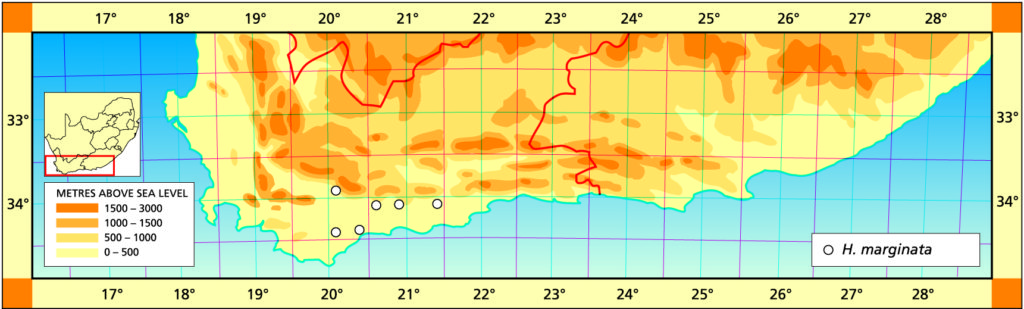
The list of names given below cannot be placed by the present author with any confidence in any presently known species. Either the descriptions are so brief or incomplete as to make identification impossible, or illustrations cannot either be allied with known species. In many cases the names are known to be, or suspected to be, referable to garden hybrids.
H. affinis Bak., JLinn.Soc.Bot. 43:213(1880). H. bilineata var. affinis (Bak.) V.Poelln., Feddes Repert.Spec.Nov. 44:235(1938). Type: Cape, McGibbon. Not preserved: See H. bilineata Baker.
H. altilinea Haw., Phil.Mag. 44:301(1824). Type: Cape. Not preserved: Baker (Flora Capensis, 1896), included H. mucronata Haw., H. limpida Haw. and H. aristata Haw. all under this name and probably incorrectly so. Those three species were described simultaneously in 1819. The origin of H. altilinea was uncertain as Haworth only indicated a certain Parmentier as the donor. Von Poellnitz also placed H. mucronata incorrectly as a variety under H. altilinea, only subsequently recognising the correct priority of names. He identified typical forms from Stockenstroom, Redhouse, Cathcart, Zwartkops (Zwartberg?) Mountains at Prince Albert, Hankey, and Port Elizabeth. There is no obvious candidate species with such a distribution range and neither can the original description of a species with characteristic raised lines on the leaves be allied with a known species. In view of the plethora of varietal epithets with similar confused geographical affinities and uncertainty concerning the application of the name, H. altilinea must be rejected as a source of confusion. Col. Scott has applied this name in lieu of H. cooperi as used in this work, and this underscores the point that there are other solutions.
H. altilinea var. bicarinata Triebn., Feddes Repert.Spec.Nov. 45:170(1938). Type: Cape, Napier, Rossouw in Triebn. 1059. Not preserved: Plants from the same collection were named H. rossouwii V.Poelln. This variety is inadequately described and is confused with H. rossouwii, which is a synonym of H. mirabilis.
H. altilinea var. brevisetata V.Poelln., Feddes Repert.Spec.Nov. 41:194(1937). Type: Cape, Vanwyksdorp, STELL6690. Not preserved: Also described from several conflicting localities, von Poellnitz in the same year reduced this variety to synonymy with H. mucronata Haw. where it probably belongs.
H. mucronata var. polyphylla fa minor (Triebn.) V.Poelln. Feddes Repert.Spec.Nov. 49:30(1940).H. altilinea var. polyphylla fa minor Triebn., ibid. 45:170(1938). Type: Elandskop, Adelaide, Triebn. 1081. Not preserved: It is quite obvious from the origin that this element is connected with the H. cooperi/H. cymbiformis complex. A recent collection from the recorded locality is cited under H. gracilis.
H. mucronata var. polyphylla fa setulifera (Triebn.) V.Poelln., Feddes Repert.Spec.Nov. 49:30(1940). H. altilinea var. setulifera Triebn., ibid. 45:170(1938). Type: Cape, Port Elizabeth, Mrs King in Triebn.911/38. Not preserved: A yellowish‑green variety with marginal teeth. It is probable that it may have some association with H. cymbiformis which does occur in that area, and none with H. mucronata.
H. angolensis Bak., Trans.Linn.Soc. 2:263(1878). = Chortolirion angolensis (Bak.) Berger. The transfer of this species back to Haworthia by A.A. Obermeyer (Bothalia 11: 119, 1973) is not accepted as it stands. Haworthia is easily subdivisible into three distinct groups and any argument re‑ordering the genera should take this into account.
H. argyrostigma Bak., Fl.Cap 6:341(1896). H. attenuata var. argyrostigma (Bak.) Berger, Das.Pflanz. 38:4(1908). Type: Ex hort. Vienna. Not preserved: Baker first used this name in discussion of H. subfasciata which is also rejected here despite the possible application the name could have in place of H. kingiana.
H. aspera Haw., Syn.Pl.Succ. :90(1812). Aloe aspera Haw., Trans.Linn.Soc. 7:7:6(1804). Apicra aspera (Haw.) Willd., Hort.Berol. 5:274(1811). Astroloba aspera (Haw.) Uitew., Succ. :53(1947). Type: Cape, Masson. Not preserved: This is a name which has been taken over to the genus Astroloba despite the fact that it was clearly described as trifarious. The name has since been excluded from Astroloba on this account by Roberts-Reinecke (unpublished). Although it may well have been an Haworthia and comparable with H. nigra, doubt is overriding and the name is rejected.
H. asperula Haw., Phil.Mag. 44:300(1824). Type: Cape. Not preserved: Although illustrated in Salm‑Dyck’s Monographia this species appears to be a dreadful source of confusion. Col. Scott in Aloe 11:30(1973), and again in his revision (1982) considered this name to apply to H. pygmaea, H. paradoxa, H. magnifica, all the varieties of H. schuldtiana, and H. pubescens; while von Poellnitz applied the name to plants from Great Brak (= H. pygmaea), Bonnievale (= H. mutica), Zebra (= H. emelyae), Uniondale (= H. bayeri) and Barrydale (= H. magnifica). Haworth described the species as “the pale rough cushion” and stated that it differed from H. retusa in being dirty‑green, sparsely tuberculate and more lined on the exposed leaf‑face. Leaf margins and keel were said to be ciliate‑denticulate. Paler, scabrous forms of H. retusa are found in the Riversdale area which can just as easily be matched to Haworth’s description as any of the other names mentioned above. Serious thought was given to using the name over H. magnifica of von Poellnitz, but the history of the name gives it little credibility.
H. baccata Smith, JS.Afr.Bot. 10:20(1944). Type: Cape, Isidenge, Stutterheim, Smith 3527 (NBG): This plant was given to Mr Smith by G. Mclaren and said to be from southwest of Stutterheim. No plant of this kind is known from that area and W.E. Armstrong informed the writer that a foreman gardener of W.T. Leighton’s had collected the plant at Frazer’s Camp. A comment by Mclaren is that the plants may have been planted into the veld judging by the poor representation there. An isotype (Smith 3782, NBG) dated July 1944 agrees with Smith’s description, but a second (Smith 3782, BDL) does not. The first is referrable to H. coarctata and the second to H. reinwardtii. The name is confused and it is certain that there is no such species.
H. bijliana V.Poelln., Feddes Repert.Spec.Nov. 27:134(1930). Type: Cape, Bredasdorp, Mrs van der Bijl. Not preserved: This plant was first collected in 1928. A short while later von Poellnitz described H. fergusoniae from Grahamstown and in Feddes Repert.Spec.Nov. 41:195(1937) placed it under H. bijliana, and added the localities Springbok and Oudtshoorn. In the absence of a type it is impossible to know just to what H. bijliana refers.
H. bilineata Baker, JLinn.Soc. 18:213(1880). Type: Cape, McGibbon. Not preserved: This species and H. affinis were described at the same time. There is no knowing what these two plants really represented and so the names are discarded.
H. gracilidelineata V.Poelln., Feddes Repert.Spec.Nov. 31:84(1932). H. bilineata var. gracilidelineata V.Poelln., Feddes Repert.Spec.Nov. 44:236(1938). Type: Cape, Little Karoo, STELL5707. Not preserved: There is no record of this collection in a list kindly abstracted for the writer from the Stellenbosch accessions by Dr H. Herre. Von Poellnitz could have had little reason to place his species in synonymy with H. bilineata and by the same token it is not possible to ally it with any known field population.
H. broteriana Res., Bolm Soc.Broteriana 15:159(1941). Type: Ex hort. Lisbon. Not preserved: A garden hybrid.
H. cassytha Bak., Fl.Cap. 6:337(1896). Type: Ex hort Pfersdorf: Baker was not even certain that this was an Haworthia.
H. cuspidata Haw., Suppl.Pl.Succ. :51(1819). Type: Cape, Bowie. Not preserved: When Haworth described this species he clearly allied it with H. mucronata which he said had pointed lanceolate leaves. The current concept of this species appears to reflect Baker’s observation (1880) that it was intermediate between H. cymbiformis and H. retusa, with no resemblance to H. mucronata. The illustration in Das Pflanzenreich 4:107(1908) seems to bear this out.
H. columnaris Bak., JBot. 27:45(1889). H. pilifera var. columnaris (Bak.) V.Poelln., Feddes Repert.Spec.Nov. 44:236(1938). H. obtusa var. columnaris (Bak.) Uitew., Succ. 29:50(1948). Type: Ex hort. Kew. Not preserved: Baker related his species to H. affinis and H. bilineata and it is unlikely that von Poellnitz was correct in trying to associate it with Baker’s H. pilifera, as did Uitewaal too. Baker would surely have seen such a connection however inadequately he perceived species.
H. confusa V.Poelln., Feddes Repert.Spec.Nov. 31:83(1932). H. minima var. confusa V.Poelln., Kakteenk. :39(1939). H. tenera var, confusa (V.Poelln.) Uitew., Succ. :52(1948). Type: Cape, Willowmore, STELL630. Not preserved: Von Poellnitz expressed doubt that the reported locality at Willowmore was correct. Other than from the fact that it was a small pellucid‑spotted species with small inconspicuous bristles there is no way of relating this name to any known species. Von Poellnitz did say that Stellenbosch No. 630 was H. gracilis and it appears that a part of that collection included H. confusa. It is known that the greater Willowmore area houses the H. decipiens var. minor and H. gracilis var. viridis complex and it is possible that von Poellnitz’ species may have originated in that.
H. curta Haw., Suppl.Pl.Succ. :61(1819). H. tortuosa var. curta (Haw.) Bak. Fl.Cap. 6:336(1896). Type: Cape, Masson. Not preserved: This is another name which could perhaps be associated with H. nigra, but there is inadequate basis for a firm supposition and Baker in fact put it with H. viscosa.
H. expansa Haw., Syn.Pl.Succ. :91(1812). A. rigida DC., Pl.Gr. :62(1799). Type: Icon, DC., Pl.Gr.: A hybrid.
H. fergusoniae V.Poelln., Feddes Repert.Spec.Nov. 28: 103(1930). Type: Cape, Grahamstown, Mrs Ferguson. Not preserved: Von Poellnitz likened this plant to H. gracilis. As it was recorded from Grahamstown it could well be synonymous with that species if von Poellnitz had not also cornfused it with H. bijliana (see same).
H. ferox V.Poelln., Feddes Repert.Spec.Nov. 31:84(1933). Type: Cape, Kendrew, STELL6631. Icon. B: This species was submitted to von Poellnitz by Dr H. Herre of Stellenbosch from a collector living at Kendrew. There is a photograph of the type plant in the Botanical Museum, Dahlem which is indistinguishable from Aloe humilis. The photograph shows a plant with a large fibrous stem typically Aloe, with thick roots emerging from the absolute base of the plant stem. Col. Scott comes to the same conclusion from a specimen at Kew. An isotype is also in the Bolus Herbarium. Various attempts have been made to substitute specimens for H. ferox and these include either robust forms of H. turgida or sparsely setate forms of H. unicolor var. venteri which occur southwest of Oudtshoorn. There is a precedent here in that Aloe aristata Haw. has frequently been mistaken for an Haworthia, and after all, von Poellnitz never did see the flower of his H. ferox.
H. ferox var. armata V.Poelln., Feddes Repert.Spec.Nov. 41:200(1937). Type: Cape, Oudtshoorn STELL5708. Not preserved. This variety was recorded from Oudtshoorn and an illustration in the Botanical Museum, Dahlem appears also to be that of Aloe humilis (L.) Mill.
H. foliolosa Haw., Syn.Pl.Succ. :99(1812). = Astroloba foliolosa ssp. foliolosa (Haw.) Reinecke, ms.
H. glabrata var. concolor (S.D.) Bak., JLinn.Soc. 18:206(1880). Type: Ex hort. Not preserved.
H. glabrata var. perviridis (Salm Dyck) Bak., J.Linn.Soc. 18:206(1880). Aloe glabrata var. perviridis Salm Dyck, Monogr. 6:f13b(1849).
H. henriquesii Res., Mems.Soc.Broteriana Succ.Afr. 2:150(1941). Type: Ex hort: A garden hybrid.
H. hybrida (Salm Dyck) Haw., Revis. :51(1821). Aloe hybrida Salm Dyck, Hort.Dyck. :2(1816).
H. icosiphylla Bak., JLinn.Soc. 18:207(1880). Type: Cape. Not preserved.
H. imbricata Haw., Syn.Pl.Succ. :98(1812). = Astroloba spiralis (L.) Reinecke, ms.
H. janseana Uitew., Cact.Vetpl. 6:45(1940). Type: Cape. Not preserved: Said to have been related to forms of H. turgida. Judging from specimens in cultivation, this is considered to be a garden hybrid.
H. kewensis V.Poelln., Feddes Repert.Spec.Nov. 49:57(1940). Type: Ex hort Kew. Not preserved: Von Poellnitz said of this plant, “This certainly has come from South Africa!” Whether this statement was intended to give added weight to the validity of the species is not known, but the plant appears to have been of hybrid origin.
H. krausiana Hort. Haage & Schmidt (cit. unknown). Cultivar.
H. krausii Hort. Haage & Schmidt No. 60165 (cit. unknown). Apparently registered as a cultivar and has no significance as far as this index is concerned.
H. lisbonensis Res. et Pinto‑Lopes, Port.Acta Biol. 4:175(1946). Type: Ex hort Lisbon. Not preserved.
H. longifolia V.Poelln. in error Farden, Cact.J 8:34(1939).
H. mantelii Uitew., Succ. 20:37(1949). = H. truncata x H. cuspidata?
H. margaritifera sv. acuminata (Salm Dyck) Berger, Das Pflanz. 33:78(1908).
H. margaritifera sv. laevior (Salm Dyck) Berger, Das Pflanz. 33:18(1908).
H. margaritifera var. subalbicans (Salm Dyck) Berger, Das Pflanz. 33:89(1908). Probably the hybrid H. pumila x H. marginata.
H. mucronata var. limpida fa acuminata V.Poelln., Feddes Repert.Spec.Nov. 49:29(1940). H. altilinea var. limpida fa acuminata V.Poelln. ibid. 45:168(1938). Type: Cape, Near Adelaide, Armstrong. Not preserved: This form was also recorded from Hankey. The Adelaide locality may have been an incorrect one and it is not certain to which species this form belongs.
H. mucronata var. morrisiae fa subglauca V.Poelln. in Feddes Repert. Spec. Nov. 49:29(1940). H. altilinea var. morrisiae fa subglauca V.Poelln. ibid. 45:168(1938). Type: Cape, Carnarvon, Roux. Not preserved: Localities given by von Poellnitz are often inconsistent with geographic distribution of known species, and this is a case in point.
H. multifaria Haw., Phil.Mag. 44:300(1824). Type: Cape. Not preserved: Haworth likened this species to H. retusa but Baker (Fl.Cap. 6:349, 1896) placed it in synonymy with H. mirabilis.
H. nigricans Haw., Phil.Mag. 44:301(1824). Type: Not known: Col. Scott is possibly correct in suggesting that this was H. nigra but it is also not unlikely that it could have been one of the really dark forms of H. scabra.
H. pearsonii Wright, Kew Bull. :365(1907). This species is insufficiently known and its origin is obscure. The illustration in the Kew herbarium is very suggestive of H. marumiana. Scott suggests that it is synonymous with H. decipiens but the long spreading perianth lobes depicted in the illustration, the narrow leaves and the description ‘ciliate on the margins’, seem to belie this possibility.
H. pellucens var. delicatula Berger, Das Pflanz. 33:(1908). H. translucens var. delicatula (Berger) V.Poelln., Feddes Repert.Spec.Nov. 44:226(1938). Type: Marloth 4208. Not preserved.
H. pentagona (Ait.) Haw., Syn.Pl.Succ. :97(1812). = Astroloba pentagona (Ait.) Uitew.
H. perplexa V.Poelln., Kakteenk. 6:67(1938). Type: Cape, Grahamstown. Not preserved: F.R. Long wrote to von Poellnitz that this plant was a natural hybrid. Private communication with Miss G. Britten, Miss G. Blackbeard and Mr W.E. Armstrong indicates that the plant was collected at Howiesonspoort, Grahamstown and was the natural hybrid H. cymbiformis X H. angustifolia.
H. polyphylla Bak., JLinn.Soc. 18:213(1880). Type: Cape, Cooper. Not preserved: As with other species described by Baker, there is no certainty about the application of this name which von Poellnitz associated with H. mucronata Haw.
H. pseudogranulata V.Poelln., Feddes Repert.Spec.Nov. 41:208(1937). Type: Cape, Oudtshoorn, STELL5711. Not preserved: This species is insufficiently known. Only four plants were collected near Volmoed (Armoed), Oudtshoorn. A specimen ostensibily from Ladismith, has been seen by the writer and it is a variant of H. venosa ssp. tessellata. H. tessellata var. tuberculata was described from De Rust.
H. radula var. asperior Haw., Revis :54(1821).
H. radula var. laevior Haw., Revis.:54(1921).
H. radula var. magniperlata Haw., Revis.:54(1821).
H. reinwardtii var. minor Bak., JLinn. Soc. 18:202(1880). Type: Ex hort. Not preserved: There are many small forms of H. reinwardtii but Baker’s description cannot be used to confirm if his variety belongs here or with H. coarctata.
H. resendeana V.Poelln., Des.Pl.Life 10:225(1938). Type: Not preserved: Of doubtful origin and considered to be an unknown triploid hybrid.
H. revendettii Uitew., Cact.Vetpl. 6:44(1940). Type: Ex hort. Not preserved: A pentaploid hybrid of unknown parentage.
H. rigida (Lam.) Haw., Revis :49(1821). A. rigida (Lam.) DC. Pl.Gr. :t62(1799). Type: Icon. :t62, DC., Pl.Gr.: This is considered to have been of hybrid origin.
H. rodinii nom.nud. A name recently brought into use for apparently the very obtuse‑leaved form of H. turgida from the Gouritz Bridge, Albertinia i.e. H. turgida var. suberecta?.
H. rubrobrunea V.Poelln., Feddes Repert.Spec.Nov. 49:57(1940). Type: Ex hort. Kew. Not preserved: Only presumed to have come from South Africa, this is also taken to be an unknown hybrid.
H. rugosa (Salm Dyck) Bak., JLinn.Soc. 18:206(1880). A. rugosa Salm Dyck, Hort.Dyck. :323(1834). Type: Ex hort. Neotype: icon, 6:f9, Salm Dyck. Monogr.(1837). The long synonymy of this element is not worth the effort of resolution.
H. rugosa var. perviridis (Salm Dyck) Berger, Das Pflanz. 4:33:92(1908). As with the preceding, it is difficult to imagine how many names would have resulted had more of these horticultural accidents been perpetuated as species.
H. ryderiana V.Poelln., Des.Pl Life 9:103(1937). Type: Cape. Not preserved: Described from a plant sent to Kew by a Mrs Ryder of Durns, England and compared by von Poellnitz with his H. willowmorensis, with H. cuspidata and H. mirabilis, none of which can make sense.
H. sampaiana Res., Bolm Soc.Broteriana 14:192(1940). Type: Ex hort. Lisbon. Not preserved.
H. sessiliflora Bak., Fl.Cap. 6:353(1896). Type: Cape, Cooper. Not preserved: The recurved leaves suggest one of the retuse haworthias in which almost-sessile flowers may occur. It cannot be allied with any known species.
H. setata var. subinermis V.Poelln., Feddes Repert.Spec.Nov. 10: 150(1936). Type: Cape, Ladismith, Joubert. Not preserved: Had this variety been more fully described and more precisely located the name may have been required to take precedence over H. mucronata var. integra or H. mucronata var. inconfluens. Von Poellnitz (1938) placed it in synonymy with H. aristata at the same time as he did his H. unicolor.
H. skinneri (Berger) Res., Mems.Soc.Broteriana: Succ.Afr. 3:77(1943). Type: Not preserved: The natural hybrid Astroloba muricata x H. maxima.
H. spiralis Haw., Syn.Pl.Succ. :97(1812). = Astroloba spiralis (Haw.) Uitew.
H. spirella Haw., Syn.Pl.Succ. :97(1812). = Astroloba spirella (Haw.) Uitew.
H. stenophylla Bak., Hook. Ic.Pl.(1874). = Chortolirion angolensis (Bak.) Berger.
H. stiemiei V.Poelln., Feddes Repert.Spec.Nov. 44:227(1938). According to Col C.L. Scott (private communication), Mr Stiemie had no knowledge of such a species or of having collected such a plant. It is possibly a variant of either H. translucens or H. arachnoidea var. xiphiophylla which occur in areas neighbouring Kirkwood, where H. stiemiei was purported to have been collected.
H. subattenuata (Salm Dyck) Bak., JLinn.Soc. 18:205(1880).
H. subfasciata (Salm Dyck) Bak., J.Linn.Soc. 18:204(1880).
H. subrigida (Roem.et Schultes) Bak., JLinn.Soc. 18:201(1880).
H. subulata (Salm Dyck) Bak., JLinn.Soc. 18:206(1880).
H. tauteae Archibald, Fl.Pl.Afr. 2S:992(1946). Type: Cape, George, Taute (GRA). This is a natural hybrid H. viscosa x H. scabra which was found at Kleinspoort east of Oudtshoorn.
H. tenuifolia Engl., Bot.Jahrb. 10:2(1889). = Chortolirion angolensis (Bak.) Berger.
H. tisleyii Bak., JLinn.Soc. 18:208(1880). Type: Not preserved: A form of H. attenuata possibly resembling this element has been recorded from near Enon by P.V. Bruyns.
H. tortella Haw., Suppl.Pl.Succ. :61(1819).
H. tortuosa Haw., Syn.Pl.Succ. :90(1812).
H. tortuosa var. major (Salm Dyck) Bak., Fl.Cap. 6:336(1896).
H. tortuosa var. pseudorigida (Salm Dyck) Berger, Das Pflanz. 33:79(1908).
H. triebneriana var. lanceolata Triebn., Feddes Repert.Spec.Nov. 47:10(1939). Type: Not preserved: Here there is also a double and conflicting habitat citation, viz. Gouritz River, Albertinia and Wolfkloof, Robertson. H. mirabilis grows at neither of these places, nor is there any other element common to both places.
H. triebneriana var. nitida V.Poelln., Feddes Repert.Spec.Nov. 49:28(1940). Type: Cape, Bonnievale, STELL7782. Not preserved: There is therefore some doubt that it belongs with H. maraisii rather than with H. mirabilis.
H. species var. typica. Current botanical nomenclature makes provision for the repetition of the species name if a variety is described. For example if the name H. glauca var. herrei is established, then automatically, the name of the typical variety H. glauca var. glauca is established. The old‑fashioned use of H. glauca var. typica is not permitted.
H. uitewaaliana V.Poelln., Cact.Vetpl. 5: 115(1939). Type: 48 km east of Riversdale, Fouche. Not preserved: It is quite probable that this was an almost glabrous form of H. minima or perhaps a hybrid H. minima x H. marginata. The latter species is, however, not known from that far east of Riversdale.
H. walmsleyi Hort. Haage and Schmidt No. 58129 (cit. unknown). Cultivar.