6. THE CAPSULES. Figs 6.1 first size differences that can be found within individual inflorescences. The remaining images show 8 capsules per population for a few populations to show variation in size and shape. The way the capsule ripens and splits is very variable. In some cases the capsule is pinched near the end but the locule tips flare outwards. This has the effect of seeds being retained in the capsule. In others the locules flare regularly and symmetrically from the base and the seed is all easily released. In the Van Reenen Crest populations the capsules were inclined to be a reddish hue. But colour can vary depending on the ripening process and they also bleach with time. Fig.6.4 7978 shows this in capsules drying after the peduncle was taken, and retaining their greenish colour. In H. floribunda the capsules are smaller and it appears that they may flare at the tips more in splitting and be coarsely crispate. This is not always the case but it is a tendency in the smaller capsules to do this. Fig. 6.7 is of capsules in 7910 H. floribunda, Rietkuil; compared against 7913 H. mirabilis also Rietkuil east of Swellendam. It is quite evident that even a capsule structure as apparently characteristic as in H. floribunda, is replicated in a different species. Fig. 6.8 is of 7955 Van Reenens Crest, and 7262 south of Greyton. I thought the capsules of 7955 were smaller, reddish coloured and slightly rougher than those of 7262. But the capsule structure in the entire genus is very similar to those shown on this figure and it is just not conceivable that some feature will stand out and resolve issues that exist in respect of the entire group.


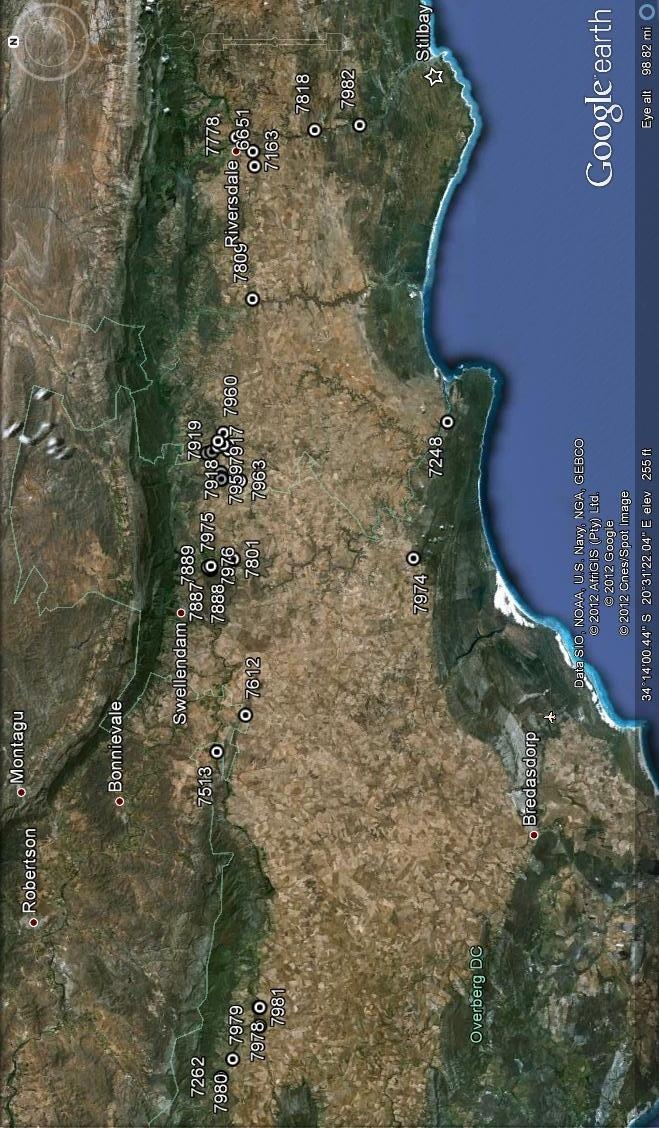
7163 H. mirabilis, S Riversdale. 7809 H. mirabilis, Koeisekop.
Fig. 6.1 Size variation – range 5-12mm.



6651 H. mirabilis, SW Riversdale
7163 H. mirabilis, S Riversdale
7778 H. mirabilis, Komserante


7818 H. mirabilis, Windsor
7809 H. mirabilis, Koeisekop,
Fig. 6.2 H. mirabilis – Eastern Zone, Riversdale area.



7913 H. mirabilis, Rietkuil.
7919 H. mirabilis, Van Reenens Crest.
7959 H. mirabilis, Van Reenens Crest.
Fig. 6.3 H. mirabilis – Central Zone, Swellendam area.


7262 H. mirabilis, S Greyton. 7978 H. mirabilis, Schuitsberg
Fig. 6.4 H. mirabilis – Eastern Zone, Greyton area.


L(1) 7888 H. mutica, Rotterdam.7910 H. floribunda, Rietkuil
R(2) 7889 H. mutica, Rotterdam.
Fig. 6.5 H. mutica – Buffeljags.Fig. 6.6 H. floribunda – Swellendam.


Above 7910 H. floribunda, Rietkuil. Above 7955 H. mirabilis, Van Reenens Crest.
Below 7913 H. mirabilis, Rietkuil. Below 7262 H. mirabilis, S Greyton.
Fig. 6.7 Some capsule comparisons.
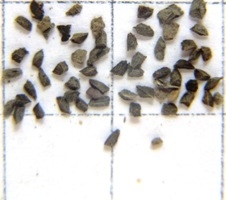
7. THE SEEDS. Some seeds are illustrated in figures Set 7. Any differences there might be in the seeds will be extremely difficult to quantify or describe. There is clearly some difference in size but to establish this statistically will be difficult. In the set of pictures provided there is clearly no difference in appearance or size coupled with the fact that the shape beyond “angular” and colour “grayish black” is difficult to articulate. What size difference appears here is due to picture magnification. Again I am quite aware that in some populations seeds are indeed larger than in others, but vegetative characters are already enough to distinguish those. Species recognition would only be facilitated if one based the definition of the species on some formula specifying seed size and shape. In the figure, 7983 is the seed of H. minima to show the quite different flattish shape and marginal wings in that subgenus. My impression was that the seed in H. floribunda may be a little chunkier than in the other samples but it just does so happen that in some capsules there are fewer and larger seed than in others.



1 – 6651 H. mirabilis, 1 – 7163 H. mirabilis, 1 – 7778 H. mirabilis,



1 – 7809 H. mirabilis, 1 – 7818 H. mirabilis, 2 – 7912 H. mirabilis



2 – 7913 H. mirabilis, 2 – 7919 H. mirabilis, 2 – 7959 H. mirabilis



3 – 7262 H. mirabilis 3 – 7978 H. mirabilis 3 – 7980 H. mirabilis



4 – 7888 H. mutica, 4 – 7889 H. mutica, 5 – 7910 H. floribunda


5 – 7963 H. floribunda, 6 – 7982 H. mirabilis, L.7262 H. mirabilis, R.7955 H. mirabilis


L.7910 H. floribunda, R.7913 H. mirabilis
7283 H. minima
SET 7 – SEEDS
DISCUSSION AND CONCLUSIONS:
While assembling and sorting the data sets I was made painfully aware of how necessary it was to keep tabs on each item. This because the similarities of between structures in very different populations was so close that without label identification, it was not possible to re-sort images into original order. Starting with flowering time that I have not yet mentioned here, as I have been dealing essentially with populations flowering in February. The southwestern Cape species of the subgenus Haworthia flower either in spring or late summer. This is the essential difference between what I regard as H. retusa (sensu lato – in the broad sense to include H. turgida) and H. mirabilis (also sensu lato to include H. magnifica, H. maraisii and H. heidelbergensis). The former set of populations flowers in the spring and the latter in late summer. But the actual time is a bit variable too as I have seen populations of the spring flowerers doing so as early as June and as late as October, while the summer group can flower from November to April. In addition there are odd plants that just flower ‘out of season’ and field hybrids present within populations of the two sets is evidence of this. It is evident from distribution, vegetative appearance and even flowering time that the two species H. retusa and H. mirabilis are parental in the origins of H. pygmaea in the east (Mossel Bay) and H. mutica in the west (Caledon). So the data here really only addresses the issue of H. mirabilis and its internal relationships, and its relationship to H. mutica in the northeastern populations. This is where one population (viz. 7801) is claimed to be a distinct species viz. H. groenewaldii. The one population 7778 H. mirabilis at Komserante obviously judging from the vegetative appearances, is infused with H. retusa. It is instructive then to look at the only two flowers I have of two nearby populations of H. retusa viz. 7780 and 7781 (see subset 6 of set 5a and 5b)
To what extent this data will influence this last mentioned claim is unknown. I simply have not and cannot discuss the floral structures in detail. This is primarily because they are so incredibly variable. I find virtually the same detail in any of the sets of figures I care to consider. What I would say that my data reflects is the interaction and continuities that I maintain are mirrored in the overall physical appearance of the plants. I have never considered 7801 H. mutica, Buffeljags as anything less than new or as anything more than the expected connection between the original H. mutica var. nigra (now H. retusa var. nigra, south of Heidelberg) and true H. mutica from the wider Bredasdorp and Caledon areas. What I did speculate was the possible involvement of H. floribunda given the distribution of that species and its interaction with both H. retusa and H. mirabilis throughout its distribution range.
I am sure this limited data set, illustrates the possible truth of my rationalization of the relationships of the plants considered. I am simply unable to extract any single point by which I can circumscribe even one population. A possible exception may be 7248 H. mirabilis, Ballyfar in which the colour yellow seems to dominate. But I recall my early involvement in Haworthia when I thought I could possibly separate H. mirabilis and H. maraisii on the basis of their flowers; with the latter having smaller and greener flowers. However, I could not do this an observations on more populations suggested to me this was just geographic variation – NOTE – differences may simply be characeristics of a species and not necessariiy denote different species. This may even apply to flowering time.
My conclusion confirms what originally impressed me. Generally flower characters will not resolve the difficulties of species circumscription based on the method, way of thinking, motives and rationale that has prevailed since Haworthia were first observed and described. Flower morphology is supposed to be central to the Linnaean method in view of their significance in the breeding process. At Buffeljags, the geographic centre of my ‘study’ area, there are close populations of H. mutica, H. mirabilis and H. floribunda across a distance of less than 600m. The plants flower synchronously and the same pollinator is present and was observed at virtually all the populations included in the entire study. H. minima and H. marginata also both occur and hybridize there too. I have up to now concluded that H. floribunda is in any case deeply infused to the point of merging into H. mirabilis both south of Heidelberg and around Swellendam. This new information, including a population pictured and reported by J. Duncan of Kirtsenbosch (nearby but within the Bontebok Park), confirms that H. floribunda is definitely interactive with H. mirabilis. My original perception of 7801 H. mutica, Buffeljags, was that it originates in the interbreeding of the three species H. retusa, H. mirabilis and H. floribunda. BUT this is not in the sense of a post speciation event where the three elements have arrived progressively at individuation as species. It is the result of the large-scale and all-encompassing process by which one gene pool has hived off H. retusa and H. mirabilis and these in turn have hived off H. pygmaea and H. mutica. The argument that 7801 H. mutica, Buffeljags is nearer to H. mirabilis than it is to H. retusa has no merit. I have in the Update Volumes explained that H. mutica is very closely allied to H. mirabilis as evident from variants seen in the field. In any case the argument rests on a typological approach where very narrow limits are attached to species names. My concept of species has developed from the plants as I have experienced them in the field, and I have not imposed any predetermined species concept on them. This study gives strength to my argument that species are complex chaotic fractal systems and that this is the model on which classification and nomenclature must be based.
(Note – a method to get more accurate mensuration would be to photograph at fixed focal length, and photographing also a millimeter rule at the same setting as I have done. However, the pictures must then be used unedited and at higher magnification more precise measurements will be attainable).
ACKNOWLEDGEMENT:
I would like to thank Lawrence Loucka for his interest in the subject and his comments and advice on the manuscript. Jannie Groenewald also accompanied and assisted me in the field, as did both Lawrence and Kobus Venter. Landowners were generous with permission and I particularly acknowledge Hesphia and Trevennan Barry of Van Reenens Crest, Nelis Swart of Postdal (Kruiskloof), Leanne and Pieter Urschel of Klipfontein, Johannes and Wilmien van Eeden of Windsor, Mathewis Odendaal of Diamant, Mark and Ryno Stander of Komserante, Wimpie and Nelie Jacobs of Koeisekop, S. Smuts of Nethercourt and P.G. Viljoen of Schuitsberg.
References:
Bayer, M.B., & Manning, J.C. Rationalization of species names in Haworthia. 2011. Haworthia Update Vol 7, part 4. Alsterworthia.
Daru, B. H., Manning, J.C., Boatwright, J.S., Maurin, O., Maclean, N., Kuzmina, M., & van der Bank, M. 2011. Phylogeny of the subfamily Alooideae (Asphodelaceae): Paraphylly of Aloe and Haworthia and consequences for classification. In ms.
Treutlein, J., Smith, G.F., van Wyk, B.E. & Wink, M. 2003a. Evidence for the polyphyly of Haworthia (Asphodelaceae subfamily Alooideae: Asparagales) inferred from nucleotide sequences of rbcL, atkK, ITS1 and genome fingerprinting with ISSR PCR. Pl.Biol. 5:513-521.
Treutlein, J., Smith, G.F., van Wyk, B.E. & Wink, M. 2003a. Phylogenetic relationships in Asphodelaceae (ubfamily Alooideae) inferred from chloroplast DNA sequences (rbcL, matK) and from genomic finger-printing (ISSR), Taxon 52:193-207.
Rhamdani, S., Barker, N.P. & Cowling, R.M. 2011. Revisiting monophyly in Haworthia Duval (Asphodelaceae): incongruence, hybridization and contemporary speciation. Taxon 60: 1001-1014.
APPENDIX 1.
Haworthia flowers – some comments as a character source.
I am adding images of flowers from 3 populations of H. mirabilis from the Worcester/Robertson Karoo. These are:-
6509 H. mirabilis meiringii , W of Bonnievale.
7176 H. mirabilis maraisii, Agter Vink.
7279 H. mirabilis meiringii, Edendale, E Bonnievale.
(The second epithet should not be taken seriously while the omission of a rank viz. var. is abandoned as is often done in zoology – I take the second epithet loosely to refer to a range of variants with some approximate but vague order).
These are to be seen as outgroups to the main body of the report/essay as show the differences of both size and colour that I once thought separated H. maraisii from H. mirabilis. Further exploration and experience have made me change my mind and I cannot see any way in which H. heidelbergensis, H. magnifica and H. maraisii can be visualized or rationalized as distinct from H. mirabilis. The flowering time for these three populations seems to be later than for the eastern and southern populations, but indeed there are populations in the MacGregor area that flower as early as November. Populations towards Montagu may flower in April. While the flowers in the populations of the main set were in seed at end March, capsules were only forming in these added three populations. A population 1708 north of Ashton was just producing buds when these were flowering as fully as could be expected in a very dry season. The flowers are indeed generally smaller than those observed in the main body but I am not sure if this is statistically certain. The same bee pollinator was observed but not the Bombylid flies. The flowers particularly in 7279 appeared to have been punctured or damaged along the sides of the flower tube as though by an insect (pollinator?) of some kind. Damage to the buds and petal ends was also noted.
I would like to explain and emphasize that I never ignored flowers as a character source and am not presenting them now as a solution to anything. What they do provide is another source of information and digital photography offers a means to capture that. It would be very silly to underestimate the volume of data required and ignore the rigours of statistics if sense and information is to be extracted from such a source. There are already problems in similarity of the flowers in what must surely be very divergent (geographically and vegetatively) species and to expect to then find differences between elements that are otherwise similar is very optimistic if not plain fantasizing. The similarity of the flower of what I named H. pulchella globifera to that of H. cymbiformis(cooperi?) var incurvula never ceases to amaze me. Similarly I could not find differences in the flowers of H. angustifolia, H. monticola, H. chloracantha and H. variegate. Another similarity was an exact copy of a flower of H. mutica and that of H. pygmaea. One aspiring taxonomist has claimed that studying the flowers of the Kaboega population set (all H. cooperi to my mind) will resolve that species issue. I think this is also a fantasy.
1. THE PLANTS.


6509 H. mirabilis meiringii, W Bonnievale


6509 H. mirabilis meiringii, W Bonnievale


7176 H. mirabilis maraisii, N Agtervink


7176 H. mirabilis maraisii, N Agtervink


7269 H. mirabilis meiringii, Edendale, E Bonnievale


7269 H. mirabilis meiringii, Edendale, E Bonnievale










































6509 H. mirabilis meiringii, W Bonnievale










































6509 H. mirabilis meiringii, W Bonnievale






























7176 H. mirabilis, Agtervink






























7176 H. mirabilis, Agtervink

























7269 H.mirabilis meiringii, Edendale, Bonnievale
























7269 H.mirabilis meiringii, Edendale, Bonnievale
APPENDIX 2.
This appendix illustrates plants and flowers and buds for the following populations:-
7985 H. mirabilis, Sandberg, S Worcester 6668 H. mirabilis, N Ashton
6672 H. mirabilis, Koningsrivier, Robertson 6875 H. mirabilis, Cogmanskloof
7986 H. mirabilis, E Montagu
Both the Sandberg and Montagu populations flowered very poorly. The Ashton and Cogmanskloof populations are known to be late flowering and I have observed this previously for a Montagu population. The Koningsriver plant is a single clone that I have had for many years.
BUDS:



7985 H. mirabilis Sandberg, 6672 H. mirabilis S Worcester Koningsrivier, 7986 H. mirabilis Robertson E Montagu


6668 H. mirabilis, N Ashton 6875 H. mirabilis, Cogmanskloof
PLANTS:


7985 H. mirabilis, Sandberg, S Worcester


7985 H. mirabilis, Sandberg, S Worcester


6672 H. mirabilis, Koningsrivier, Robertson


6672 H. mirabilis, Koningsrivier, Robertson


7986 H. mirabilis, E Montagu


7986 H. mirabilis, E Montagu


6668 H. mirabilis, N Ashton


6668 H. mirabilis, N Ashton


6875 H. mirabilis, Cogmanskloof


6875 H. mirabilis, Cogmanskloof
APPENDIX 3:
5b.1 7910a 5b.1 7910b
H. floribunda, Niekerkshek – size comparisons
5b.2 7980a 5b.2 7980a
H. mirabilis, S Greyton – size comparisons
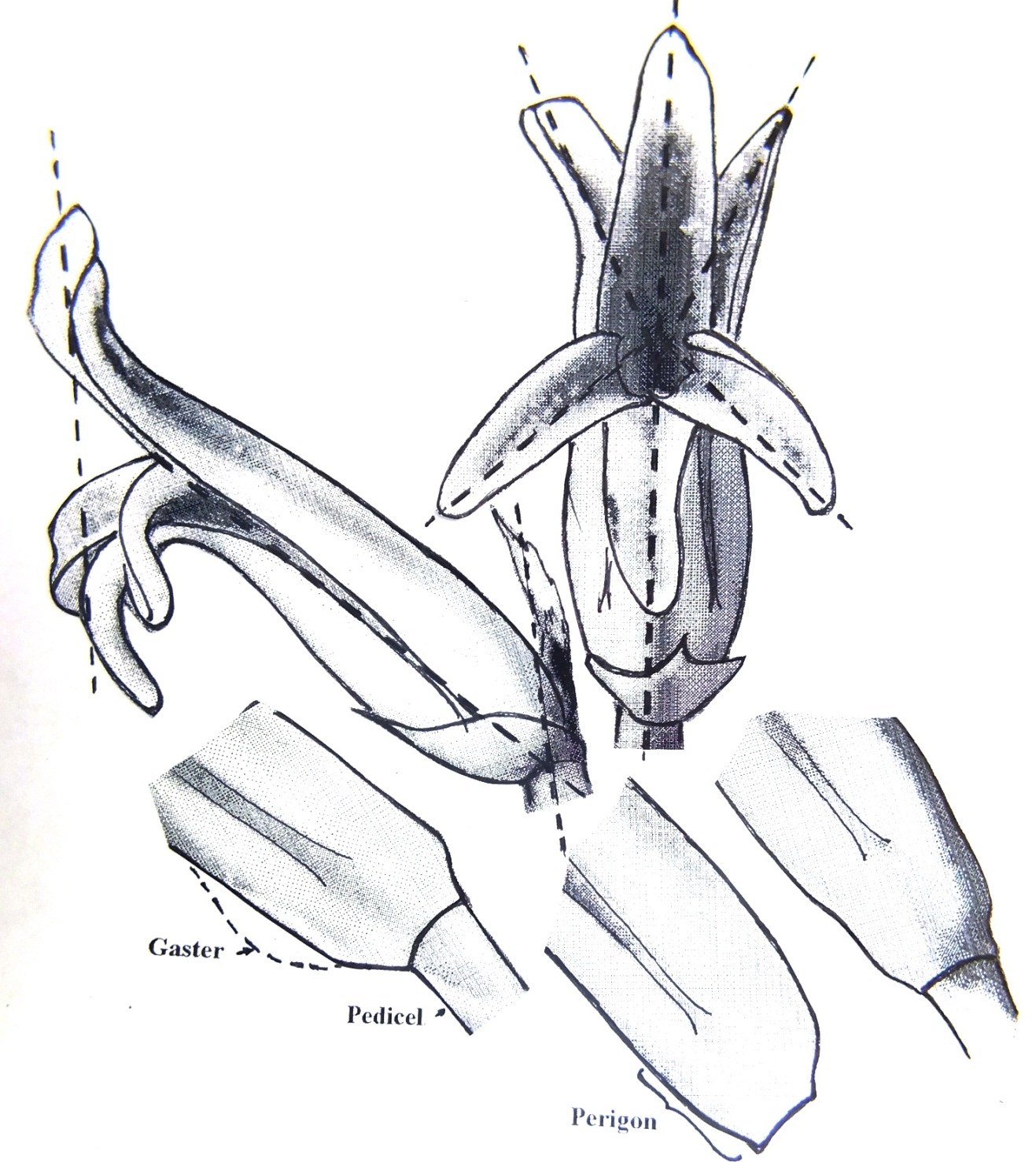
Diagram of flower to show face and profile. The base of the tube is how they can vary in one population. The perigon may narrow gradually stipe-like to the pedicel, or abruptly. The tube may have a pronounced gaster as in Gasteria or the profile may be smoothly curved with no broadening beyond the perigon. The outer leaf margins may meet or be separated to expose the inner midribs. The basal separation of the petals may be quite deeply indented or relatively even.
The dotted lines are suggested as base lines for possible mensuration and computer modeling.
Haworthia flowers – some comments as a character source, part 1
Haworthia flowers – some comments as a character source, part 2
Haworthia flowers – some comments as a character source, part 3

Pingback: Haworthia flowers – some comments as a character source, part 1 - Haworthia Updates
Pingback: Haworthia flowers – some comments as a character source, part 2 | Haworthia Updates