Printed in Cactus and Succulent Journal (US), 43:157 (1971).
M.B.Bayer, National Botanic Gardens of South Africa, Karoo Garden, Worcester.
The genus Haworthia is sub-divided into twenty sections principally on leaf and growth habit. The composition of the sections thus often bears little relation to the actual and natural affinities of the constituent species. Sub-division of the genus on the basis of floral characters has been suggested by Uitewaal (1947) and reference should also be made to Berger (1908). In Das Pflanzenreich, under the Section Margaritiferae, Berger particularly comments (citing N.E. Brown) on the problem of garden hybrids, and it is unfortunately true that many species are still recognized which have no real basis in field populations. These inevitably obstruct attempts at presenting natural relationships, and hence also a desired aim of plant classification. The inadequacies of leaf morphology as a basis for subdivision is particularly evident in a study of the species H. reticulata Haw., H. herbacea (Mill.) Stearn and H. schuldtiana var. maculata vPoelln.. These species are presently placed in the sections Muticae, Arachnoideae, and Retusae respectively, when in fact distribution, inherent variability, field hybridization and floral morphology demonstrate their unquestionable affinity.
The section Margaritiferae is particularly afflicted with dubious species and there is some doubt that the species H. icosiphylla Baker, H. tisleyii Baker, H. rugosa (S.D.) Baker, H. .cuspidata (S.D.) Baker, H. subattenuata (S.D.) Baker and H. subfasciata (S.D.) Baker can be referred to field populations and thus validated. On the basis of floral morphology, the species H. attentuata Haw., H. browniana vPoelln, H. fasciata Willd., H. glabrata Baker, H. longiana vPoelln. and H. radula Haw. (if these are actually discrete species), together with H. smitii vPoelln.and H. tuberculata vPoelln. whose affinities are less obvious, can be transferred to the section Coarctatae. Examination of preserved material in the Bolus and Compton Herbaria (Cape Town), collecting records of Smith, Otzen, Long and Payne, and direct field observation indicate that only four species need to be considered under the Margaritiferae. The following account is given in explanation of this statement.
The described varieties and related species of H. margaritifera (L.) Haw, are particularly confused and it is fortunate that field populations do not make it necessary to fully clarify the involved synonymy of this species. Commelin (1701) recognized two species, Aloe africana folio in summitate triangulari margaritifera flore subviridi (t.10) and Aloe africana margaritifera minor (t.1l). In 1715 he added two more species, one merely with fewer tubercles and probably synonymous with both his t10 and t.11, and Aloe africana margaritifera minima (n.43) which was described as having smaller leaves and tubercles than the variety minor. Boerhaave (1720) cited four related species but referred only to two of Commelin’s earlier species. Bradley (1725) described and illustrated a plant which he vaguely referred to Boerhaave. This is unfortunate because although Bradley’s illustration (t.21) is clearly intended to be the same as Commelin’s minima, it became a source of confusion in later works. Dillenius (1732) elaborated on Commelin’s species and provided illustrations for separation of the elements minor (f.17) and minima (f.18).
Linnaeus recognized three elements which were based on Commelin’s t.10, t.11 (Dillenius’ f.17) and n.43 (Dill. f.18), Aiton (1789), in retaining three elements, cited only Bradley’s t.21 under his Aloe niargaritif era var. major and yet under the variety minima also refers to Bradley’s species! DeCandolle (1799) illustrated a plant (t.57) as Aloe margaritifera and this was said by Ker-Gawler (1811) to really have been the variety minor. DeCandolle had used the epithet media instead of minor, and as Ker-Gawler (1805) had repeated the error it is not surprising that Aiton (1811) added Decandolle’s t.57 and KerGawler’s media to the synonymy of his Aloe margaritifera var, minor. It is perhaps Aiton’s earlier treatment of Bradley’s t.21 which prompted Haworth (1805) to recognize four elements, namely Aloe maxima (type Commelin t10), Al. minor (type Comm. t.11 and Dill. f.17), Al. minima (type Comm n.43 and Dill. f.18) and Al. major (type Bradley t.21).
Willdenow (1811) appears to have been confused as he described Apicra margaritifera var. major (type Comm. t.10 and Bradley t.21) and var. minor (type Dill. f.18). He also described Ap. granata (type DeCandolle t.57) and Ap. margaritifera var. maxima (no syn.). This latter species may have been intended to be Haworth’s Al. maxima. Salm-Dyck (1817), however, altered Haworth’s Haworthia maxima to Aloe semimargaritifera with three varieties major, minor and minima, and described Al. papillosa based on Willdenow’s Ap. margaritifera var. maxima. It seems fairly certain therefore that Al. papillosa S.D. and H. maxima Haw. were synonymous. Saim-Dyck retained Al. margaritifera and also its varieties major, minor and minima.
Haworth (1819) now totally revised his species by accepting Salm-Dyck’s Al. semimargaritifera and adding a fourth variety multiperlata. He altered his H. margaritif era var. major to the species H. margaritifera, retained H. minor, described H. erecta (type DeCandolle t.57) and H. brevis (type Ker-Gawler t.1360), and altered his H. minima to H. granata. He also discarded his H. maxima in favor of H. papillosa. The species H. semiglabrata was also described.
Some rationalization has taken place with time and the following species and varieties arising from the above are presently recognized : –
H. papillosa (S.D.) Haw.
Hf. margaritifera var. margaritifera (L.) Haw. var, corallina Baker
var. maxima Haw.
var. minima (Aiton) Uitew.
var. minor (Aiton) Uitew.
var. subalbicans (S.D.) Berger
In addition there are several subvarieties and also the related species H. semiglabrata Flaw., H. poellnitziana Uitew., H. uitewaaliana v Poelln. and H. mutabilis v Poelln.
Field observation clearly demonstrates that all these taxa are misconceived and that historically, confusion revolves around two principle elements. These are firstly the species typified by both Commelin’s t.1O and t.11, found in the Robertson Karoo and western area (Montagu) of the Little Karoo. This is H. margaritifera (now H. pumila). Secondly there is the species found in the Coastal Renosterbos and Machia veld-types and typified by Commelin’s n.43 (Dill. f.18). This is H. margaritifera var. minima and upheld by the writer as a distinct species, H. minima Haw. This species is not to be confused with Baker’s illegitimate H. minima for which the name H. tenera v Poelln. was upheld by Uitewaal.
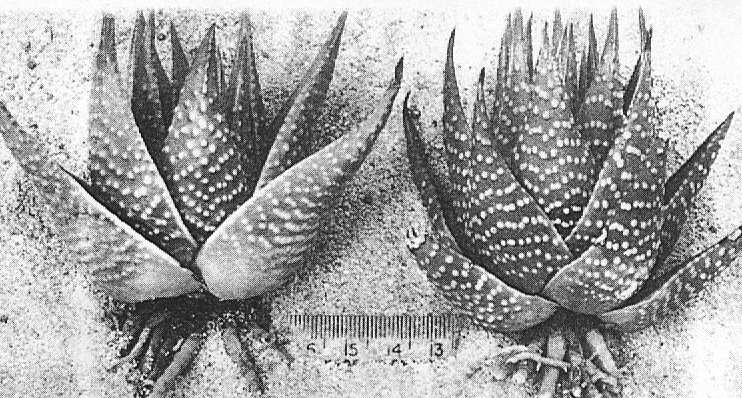
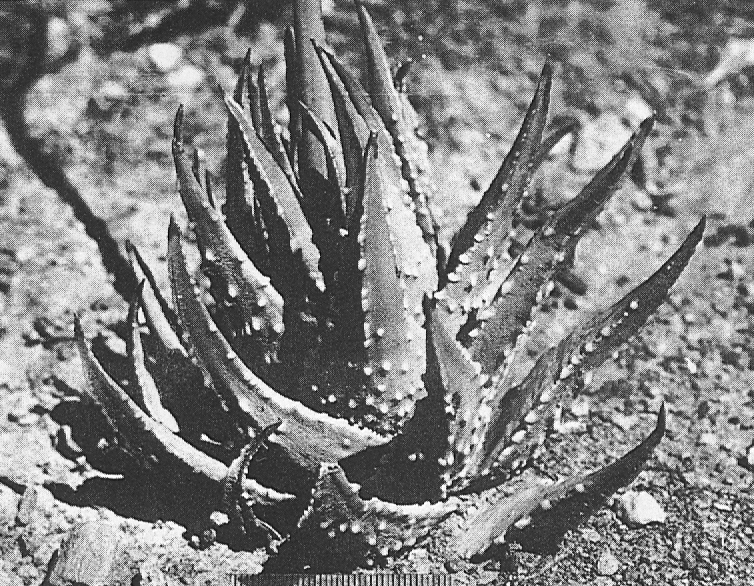
H. poellnitziana was investigated at its type locality at Drew, and a related population was also examined in the same ecosystem some three miles to the north-west. The type locality is a very restricted area and Mr. G.J. Payne communicated the information that H. marginata var. virescens (Haw.) Uitew. had previously been determined from this same site. Some specimens collected at this locality by the writer showed evidence of smoothness typical of H. marginata (Lam.) Steam and the presence of hybrids of this species with H. niargaritifera nearby at Ashton indicate that H. poellnitziana may be of hybrid origin. The population to the northwest can either be considered to be a larger form of H. minima more like those found in the inland areas from Riversdale to Swellendam, or to be an ecotype of H. margaritifera adapted to the sandy soils of the Coastal Renosterbos veld-type. The problem is illustrated in the accompanying photographs. For the present, H. poellnitziana can be regarded as a variety of H. miargaritifera. Plants and seed from the type locality are in cultivation at the Karoo Garden and these will permit a final diagnosis at a later date.
H. papillosa can be dismissed as a variant of H. margaritifera with little further ado. Apart from having its origin in Commelin’s t.1O, field observations show that any of the attributes by which this species can be considered discrete, are fallacious. Under good conditions H. margaritifera may grow enormous and often develop an elongated leafy stem. The density of tubercles is in any case variable and it is not uncommon to find the leaf faces smooth either. In some specimens the leaves are arranged in conspicuous spiral fashion also.
The discovery of H. mutabilis vPoelln. is attributed to G.J. Payne who recorded it from the farm Mierkraal, south-west of Bredasdorp (also the type locality of H. mundula Smith). Mr. Payne informed the writer that the species was based on an atypical specimen of H. minima, which was the only relevant species found by the writer in that area. Thus H. mutabilis cannot be upheld and is reduced to synonymy under H. minima.
H. marginata hybridizes commonly with both H. margaritifera (N. and S.E. of Ashton) and with H. minima (S. of Heidelberg). These hybrids are undoubtedly the origin of Zantner’s curious paper which appeared in Beitrage zur Sukkulentekunden (1942), the one real merit of which was the statement that H. marginata and H. margaritifera were closely associated species and needed to be considered in the same context. H. semiglabrata certainly offers no problems and specimens satisfying the description for this species are common in the hybrid swarm H. margaritiferaX marginata near Ashton. Similarly H. uitewaaliana must be dismissed as another form within a similar hybrid association, if not described from a luxuriantly growing young specimen of H. marginata.
There is some doubt as to the origin and validity of H. subjasciata S.D. The variety kingiana vPoelln, is quite distinctive and occurs in a different ecological area, notably the Karoid Valley Bushveld of the lower Great Brak River. Thus H. kingiana is upheld by the writer as a distinct species, the species subfasciata being discarded as dubious.
The four species are thus H. margaritifera, H. minima, H. marginata, and H. kingiana. All these species have a characteristic heavy branched peduncle to which Berger and N.E. Brown (1878) refer, and for which Uitewaal proposed recognition as a subdivision Robustipedunculatae of his group Hexangulares. Also characteristic of these species is the abruptly rounded attachment of the perigon onto the pedicel. This, coupled with the relatively narrowly opening perianth lobes and regular arrangement of the outer lobes, separates the four species from all other Haworthia. The large rounded seed capsules and characteristic configuration of the empty capsules are also distinctive. These all suggest a distinct component of the genus at sub-generic level together with the groups Triangulares and Hexangulares proposed by Uitewaal. The nature and degree of difference between the three subgenera so formed appears to warrant re-evaluation of at least the genera Astroloba, Chortolirion and Haworthia.
The writers concept of subdivision of the genus Haworthia is implemented as follows:-
Haworthia Duval subgenus Haworthia
Type: H. arachnoidea (L.) Duval
Plantae acaules, folia carnosus, pedunculus gracilis simplex, perianthium ad basim triangularis vel rotundatus triangularis, tubus subabruptus junctus versus pedicellus, stylus brevis flexus, stigma sursum capitatus, capsula elongatus cylindricus.c (The name Triangulares proposed by Uitewaal for this group cannot be accepted on account of Article 22 of the International Code of Botanical Nomenclature).
Haworthia Duval subgenus Hexangulares Uitewaal
Type: H. coarctata Haw.
Plantae acaules vel caulescentes, folia rigidus firmus elongatus vel plantae caulescentes folia abbreviatus incrassatus, pedunculus gracilis plerumque simplex, perianthium ad basirn hexangularis vel rotundatus hexangularis, tubus decrescens versus pedicellus, stylus elongatus erectus, stigma prorsus subcapitatus, capsula elongatus cylindricus.
Haworthia Duval subgenus Robustipedunculares Uitewaal
Type: H. margaritif era (L.) Haw.
Plantae acaules, folia rigidus incrassatus, pedunculus robustus ramosissimus, perianthium hexangularis, vel rotundatus hexangularis, tubus abruptus junctus versus pedicellus, stylus elongatus erectus, stigma prorsus subcapitatus, capsula grandis rotundatus.
The following key divides the genus into the three subgenera : –
1. a – Perianth at base triangular or rounded triangular…………………………… Subgenus Haworthia
b – Perianth at base hexangular or rounded hexangular…………………………………….. 2
2. a – Perianth gradually narrowing to junction with pedicel………………. . . . .Subgenus Hexangulares Uitewaal.
b – Perianth abruptly joined to pedicel Subgenus ……………………………Subgenus Robustipedunculares Uitewaal.
The sections as recognized at present are allocated to the subgenera almost as indicated by Brown (1957), with the exception of the section Albicantes which now falls under the subgenus Robustipeduinculares. The Robustipedunculares therefore includes the species H. margaritifera, H. minima, H. kingiana and H. marginata. The sections Albicantes and Margaritiferae can be retained in this subgenus with the exclusion of the dubious and invalid species already noted, and the exclusion of those species transferred to the Coarctatae. The further correct and natural breakdown of the subgenera will require an intensive study of both floral and leaf morphology. As already indicated, the present sections are not natural, and natural groups cannot be constructed on leaf structure and arrangement alone. For example H. marginata is separated from H. margartifera on the basis of leaf armature (glabrous and tubercled respectively) yet flower structure conclusively demonstrates their proper affinity. It is conceivable that the glabrous H. starkiana vPoelln. and tubercled H. tuberculata vPoelln, may prove similarly related. Rationalization of the differences between the four species constituting the subgenus Robustipedunculares is relatively easy. H. marginata is separated immediately by its glabrous leaf surface. The inflorescence is similar to that of H. marganitifera except that the perianth lobes are white suffused with pink. H. marginata has been recorded from north of Bredasdorp, west of Napier, south of Swellendam, Heidelberg, Riversdale and also north and southeast of Ashton. It thus occurs principally in the Coastal Renosterbos veld-type except at Ash ton where it is found in Karoid Broken veld.
H. kingiana is separated by its more brilliant green color, fewer tubercies, and tubercles which are rounded, smooth and hardly projecting above the leaf surface. Ecologically this species is separated by its distribution. It is confined to the Karoid Valley Bushveld east of Mossel Bay, although this veld type is also found in part of the Gouritz River Valley to the west.
H. minima and H. margaritifera pose slight problems. There must be doubt in view of what has been written concerning H. poellnitziana, that these two species are separable. H. minima is characteristically smaller in the coastal areas, but inland may reach a height of 10 cm. and 8 cm. diameter. Although H. margaritifera may also be small under adverse conditions, it often reaches a height of 30 cm. and 20 cm. diameter. It is also more coarsely tubercled. The leaf ground-color in H. minima is grey-green and the tubercles distinctly white, whereas in H. margaritifera the ground color is dark-green varying through to deep brick-red under adverse conditions. H. minima frequently forms dense compact clusters. H. margaritifera seldom proliferates and when it does the plants are often characterized by the visible, elongated stems with dried and missing basal leaves. The color of the outer perianth lobes is usually a distinctive yellowish-brown in H. margaritifera compared to H. minima where they are pinkish-white. Finally the perigon in the former species may be up to 4.5 mm. in diameter and seldom more than 2 mm. in the case of H. minima.
Ecologically H. minima ocurs in the Coastal Renosterbos and Coastal Macchia veld-types which are associated with sandy soils. H. margaritifera occurs in the heavier clay soils derived from Karoid shale formations, and in the associated Karoid Broken veld of the Robertson, Bonnievale, Worcester and Montagu areas. Morphological differences are maintained under cultivation in same soil mixtures and growing conditions. With those differences in mind, the correct decision appears to be separation at species level. There is no evidence to support the multiplicity of varieties and sub-varieties presently recognized, and even the variety poellnitziana would be more correctly treated as anomalous.
The sub-genus Robustipedunculares is expected to key out reliably in the following manner: –
1. a – Leaf surface smooth……………………………………….. H. marginata (Lam.) Stearn.
b – Leaf surface tubercled ……………………………………… 2
2. a – Tubercles smooth rounded, hardly raised above leaf surface… H. kingiana vPoelln.
b – Tubercles rough, distinctly raised above leaf surface………… 3
3. a – Leaf color gray-green, plants small (to 10 cm. high) outer perianth lobes pinkish-white… H. minima Haw.
b – Leaf color dark-green, plants large (to 30 cm. high), outer perianth lobes yellowish-brown…
….. H. margaritifera (L.) Haw.
Leaf color is not a reliable criterion for separation of these species as obviously under artificial conditions the usefulness of this chararater may diminish.
LITERATURE CITED
Uitewaal, A. J. 1947. A first attempt to subdivide the Genus Haworthia based on floral characters. Desert Plant Life 19; 133-138.
ACKNOWLEDGEMENT
The writer wishes to acknowledge the interest, encouragement and assistance received from Mr. F. J. Stayner, Curator of the Karoo Garden, Worcester. Mr. Colin Walker of Stockport, England, and of the Haworthia Study Group, Cactus and Succulent Society of New South Wales, Australia.
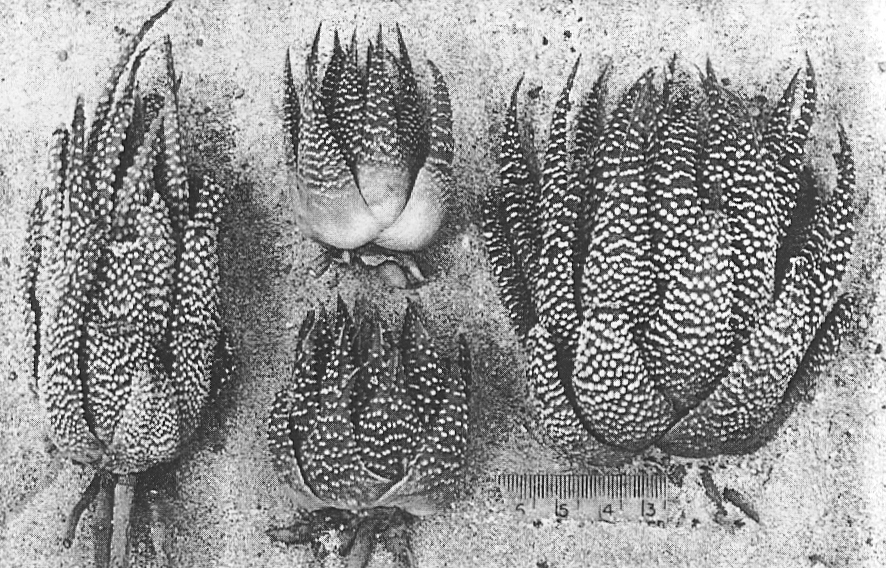
Fig. 2. Left: “H. poellnitziana Uitew.” 3m NW Drew. Centre: H. minima Haw., S Bredasdorp, small coastal form. Right: H. minima NE Riversdale, larger inland form.

Fig. 3. Left: H. margaritifera (L.) Haw., 8 m. SE of Drew. Centre: ‘H. poellnitziana’ 3 m. N.W. of Drew. Right: “H. poellnitziana” from the type locality at Drew. Note keel at leaf tips, typical of H. margaritifera x marginata hybrids.
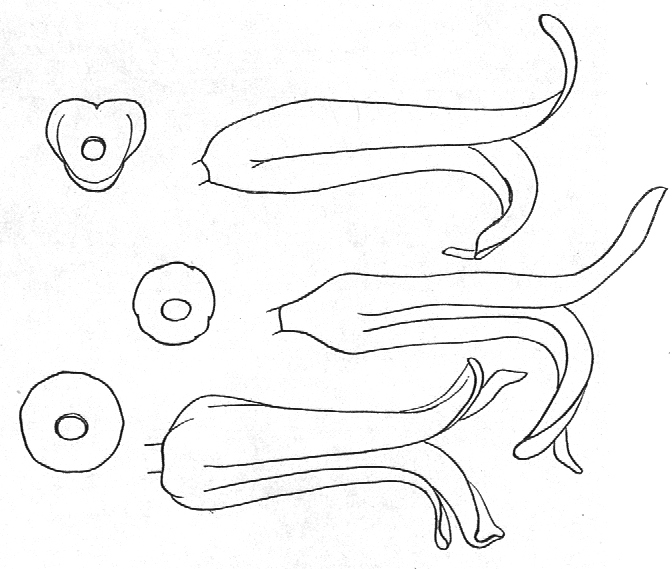
Fig. 7. Diagramatic presentation of typical base and profile of flowers in the subgenera (a) Haworthia (top, (b) Hexangulares Uitew. (middle), and (c) Robustipedunculares Uitew. (bottom).