60. Haworthia minima (Ait.) Haw., Syn.Pl.Succ. :92(1812). Bayer, Cact.Succ.J(U.S.) 43:157(1971). Bayer :135(1976). Bayer :81(1982). Scott :17(1985). A. margaritifera var. minima (L.) Ait., Hort.Kew. 1:468(1789). Willd., Spec.Plant. :189(1799). Haw., Trans.Linn.Soc. :8(1804). A. margaritifera var. minor (L.) Lam., Encycl. :88(1783). A. margaritifera (L.) Burm.(f), Prod.Fl.Cap. :10(1768). A. pumila var. margaritifera beta L., Spec.Pl. :322(1753). H. granata (Willd.) Haw. Suppl.Pl.Succ. :57(1819). Haw., Revis. :203(1821). Apicra granata Willd. Berl.Mag. :269(1811). A. granata (Willd.) Roem. et Schultes, Syst.Veg. 7:649(1829). A. granata (Willd.) Salm Dyck, Monogr. 6:t6(1836). H. margaritifera var. granata (Willd.) Baker, JLinn.Soc.Bot. 43:205(1880). A. erecta var. laetivirens Salm Dyck, Hort.Dyck. :12((1824). Type: icon. 20:t16,f18, Dill. Hort.Elth. (1732): Haworthia minor (Haw.) Duv., Hort.Alenc. :7(1809). Haw. Syn.Pl.Succ. :92(1812). Haw. Suppl.Pl.Succ. :53(1819). A. margaritifera var minor (L.) Ait., Hort.Kew. :468(1789). Haw., Trans.Linn.Soc. 7:11(1804). Ker-G., Bot.Mag. :t815(1805). A. pumila var margaritifera gamma L., Spec.Pl. :322(1753). A. erecta Salm Dyck, 6:t7(1836) Type: icon. 20:t16,f17, Dill. Hort.Elth. (1732): H. margaritifera Haw., Suppl.Pl.Succ. :55(1819). H. major (Ait.) Duval, Hort.Alenc. :7(1809). Aloe margaritifera var. major Ait., Hort.Kew. :468(1789). Haw., Trans.Linn.Soc. :8(1804). Type: icon. 1:t21, Bradley, Hist.Succ.Pl. 3(1725): H. erecta Haw. Suppl.Pl.Succ. :56(1819). Haw., Revis. :55(1821). H. margaritifera var. erecta (Haw.) Baker, JLinn.Soc.Bot.18:205(1880). A. erecta (Haw.) Roem. et Schultes :649(1829). non A. erecta Salm Dyck 6:t7(1836). A. margaritifera var media DC. Pl.Gr. :57(1799). A. pumila var. margaritifera gamma L., Spec.Pl. :322(1753). Type: icon, 21:t11, Comm. Prael.Bot. (1701): H. brevis Haw., Suppl.Pl.Succ. :57(1819). A. brevis Roem. et Schultes, Syst.Veg. 7:649(1829). A. margaritifera var. minor Ker‑G., Bot.Mag. :t.1360(1811). Type: icon, :t1360, Ker-G., Bot.Mag.: H. margaritifera var. corallina Baker, JLinn.Soc.Bot. 43:201(1880). Type: Ex hort. Peacock. Not preserved: H. mutabilis V.Poelln., Feddes Repert.Spec.Nov. 44:132(1938). Type: Cape, Bredasdorp, Payne 23 in Triebn.1110. Not preserved.
minima: small.
Rosette stemless, slowly proliferous, to 150mm tall. Leaves to 130 X 15mm, nearly as thick as wide, attenuate, spreading, lanceolate-deltoid, surfaces scabrid with raised flattened non-confluent tubercles, colour bluish-green. Inflorescence sparsely branched, lax. Flowers tepals fused, tube straight, lobes abbreviated, veins pinkish.
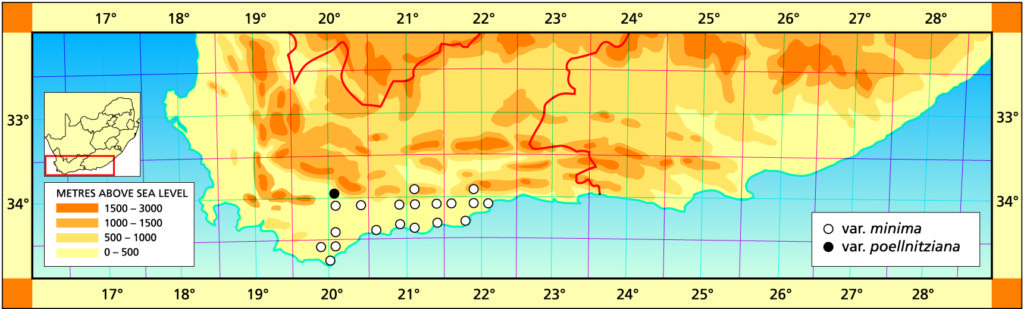
1982 – H. minima is a small (up to 120mm high), clump forming, blue‑green species, with white tubercles and pink‑tipped florets. It occurs in Coastal Renosterbos from Bredasdorp eastwards to at least the Gouritz River, and inland to Swellendam and Heidelberg. Very tuberculate forms are reported in the coastal limestones and a nearly glabrous single plant from Bredasdorp was named as H. mutabilis V.Poelln. There is no indication of any hybridisation or intergradation with either H. pumila in the west or H. kingiana in the east. The relationship with H. poellnitziana requires detailed examination. H. minima hybridises with H. marginata at Heidelberg (Cape).
1999 – The only interesting development concerning this species is the collection from north of the Langeberg Mountains. It has been found at Brandrivier to the east, and still further east, northeast of Herbertsdale. H. poellnitziana is now included as a variety of this more generally distributed species simply in recognition of popular opinion. H. minima does develop long slender leaves, but the fact remains that there seems to be a difference in flower colour, while the distribution is also disjunct. As is unfortunately usually the case, no herbarium specimens are provided to substantiate opinions. The synonymy has been substantially altered follow the views expressed concerning the correct naming of H. maxima (Haw.) Duv. My opinion has been that if the iconotypes had been properly known and understood, the nomenclature of the two species involved would have been much simpler. H. maxima would have been seen to be the correct name for the “large pearled aloe”, and H. margaritifera for the “lesser pearled aloe”. The old synonymy follows the types quite closely and unfortunately those who have attempted to pronounce on the nomenclature seem to have been under the impression that the iconotypes were mostly the larger of the species. The converse is true.
a. var. minima
Distribution: 3321 (Ladismith): Brandrivier (-CC), Laidler 707 (NBG); Perdekop, Bonniedale (-DD), Matthews 1095 (NBG). 3419( Caledon): Wiesdrift (-DB), Smith 5475 (NBG); Voelvlei (-DB), Dymond (BOL); Mierkraal (-DB), Fourcade 199 (NBG)7. 3420 (Bredasdorp): Swellendam (-AA), Rotheno in PRE 39425; Bontebok Park (-AB), Bayer & Fourie 4432 (NBG); NE. Bredasdorp (-AC), Smith 5472 (NBG); Skeiding (-BB), Smith 7220 (NBG); Heidelberg (-BB), Fouche 45 (PRE), Smith 5508, 6569, 7146 (NBG); Bassonskraal (-BC), Burgers 1333 (NBG); Koenskraal (-BC), Venter 4 (NBG); Infanta (-BC), Ross-Frames (BOL); Malgas (-BC), Esterhuysen 5224 (BOL); N. Infanta (-BD), Smith 5474 (NBG); Bontebok Park (-CA), Barker 7232 (NBG); De Mond (-CA), Smith 7352 (NBG); Struisbaai (-CC), Esterhuysen (BOL). 3421 (Riversdale): SW. Riversdale (-AA), Smith 5469 (NBG); Swartheuwel (-AA), Smith 7190 (NBG); Kruisrivier (-AB), Smith 7319 (NBG); About 3km SW. town (-AB), Dekenah 3 (PRE); E. Riversdale (-AB), Smith 5375, 5486, 5757, 6087 (NBG); Vermaaklikheid (-AC), Smith 6108 (NBG); Stilbaai (‑AD), Dekenah 97 (NBG), Smith 5504, 5283 (NBG); E. Albertinia (-BA), van Niekerk 333 (NBG); E. Gouritz Bridge (-BB), Smith 3960, 7354 (NBG); N. Gouritzmond (-BD), Smith 6101, 7518 (NBG). 3422(Mossel Bay): Hartenbos (-AA), Schoeman (NBG).
Inadequately located: Ex hort., Steyn in PRE 8797; Riversdale, Ferguson (BOL), Muir in NBG616/23 (BOL); Kafferkuilsrivier, Bolus (BOL); Bredasdorp, James (BOL).

Haworthia minima var. minima JDV 84/81 Brandrivier. Also crossing the Langeberg Mountains, this population flowers, like the one at Swellendam, in November or earlier. 
Haworthia minima var. minima JDV91/23 southwest of Heidelberg. A spectacular form which is very blue in colour. 
Haworthia minima var. minima JDV89/91 north of Heidelberg. This population is proliferous and seems to include some H. marginata influence. 
Haworthia minima var. minima JDV93/55 east of Heidelberg. Occassionally growing as single heads. 
Haworthia minima var. minima JDV89/19 north of Heidelberg. Some of the plants are less tubercled and may also be greener in colour. 
Haworthia minima var. minima JDV84/81 Brandrivier. The tubercles are usually smoother than is the case in H. pumila. 
Haworthia minima var. minima JDV96/32 southwest of Bredasdorp. Remarkably able to survive the cutting of the surrounding grass for hay.
b. var. poellnitziana (Uitew.) Bayer Comb.nov. H. poellnitziana Uitew., Cact.Vetpl. 5:137(1939). Bayer :146(1976). Bayer :81(1982). Type: Cape, Drew, Meiring. (AMD).
poellnitziana: in honour of Dr. K. von Poellnitz.
1982 – H. poellnitziana occurs on the western extremity of the range for H. pumila, and H. minima does not occur nearer than 30km to the southeast. H. poellnitziana has slender long leaves up to 180mm long. They are grey‑green in colour and less blue than in H. minima, while in H. pumila the leaves are dark brownish‑green. The flower lobes are yellowish in colour. It is in a winter rainfall area, and grows in fynbos vegetation on old river gravels.
Distribution: 3320 (Montagu): West of Drew, Swellendam (‑CC), Smith 3947 (NBG), Fourcade 185 (NBG), Bayer 154 (NBG); Robertson, van der Merwe 225 (BOL).

Haworthia minima var. poellnitziana JDV90/30 northwest of Dre. H. Pumila grows vary nearby but thre does not seem to be any hybridization, as there may be with H. marginata which is also in the area. 
Haworthia minima var. poellnitziana JDV90/30 northwest of Drew. A large robust form which is not very proliferous. Seedlings and flower colour definetly suggest the affinity with H. minima. 
Haworthia minima var. poellnitziana MBB6624 northwest of Drew. A few plants have also been located near Drew itself, but otherwise the nearest known populations of the typical variety are at Swellendam.