M.B. Bayer, with acknowledgment to P.V. Bruyns and J.D. Venter.
It is unfortunate when a situation arises where individuals compete to provide a classification for a group of plants. The normal process for aspiring taxonomists is to determine what needs to be done that is not being done, and which attracts and interests them and so select a group to study. There are probably not many instances where different people have worked comfortably together to explore and resolve the taxonomy of any plant group. The objective of this article is to show how that it should be possible to look rationally and objectively at a problem and produce a solution which can be taken forward in the same way.
I think it is understood in the taxonomic fraternity that one of the main aims of a classification is that it must presume to account for all of the plants in the group in question. Is this physically possible? Nobody in the least familiar with Haworthia has any doubt that the territory to be covered for a study of the genus is unbelievably big. The nature of habitats and size of populations, coupled with intrinsic variability and the sometimes cryptic nature of many of the plants make it not only possible but probable, that exploration of even a relatively small geographical area may miss something important. This complexity in the field is not unique to Haworthia. A classification therefore can never be considered to be complete and is only an hypothesis based on what is known. For that which is at the time unknown, it should be assumptive and predictive. It is further necessary that the classification meets the needs of a general group of people who will use it. The nature of these needs is in a large part already expressed in the preceding attempts to classify the group and in the literature associated therewith. A classification is important in the way it allows of generalisation, extrapolation to hitherto unknown collections and for communication.
The test of the hypothesis contained in the classification is the subsequent successful identification of plants by means of the key provided and also by the incorporation of new data into the structure of the classification. It can also be tested by the ease with which people communicate about the components of the classification, and, of course, whether it comes to be generally accepted at all. The strongest and most practical test is its acceptance by curators of herbaria and the way it is used to store and retrieve data in an herbarium.
In the case of plants popular in horticulture and with the general public (such as orchids and succulents) there is often controversy over their classification. The reason for this controversy is that, because of their popularity with the general public, untrained and non‑professional people are drawn into the process of classification and identification. Their only justification for this is their own enthusiasm and interest in the subject which they feel generates new and previously unrecorded information which they perceive a real need to express. It should be recognised that they do not necessarily make any more or fewer mistakes than professionals working on obscure groups or at levels of classification (or sophistication) beyond the reach and interest of the layman. There are also many cases where so-called ‘amateurs’ have made contributions unsurpassed by professionals. It should also be noted that many classifications by professionals may very seldom come under any kind of practical and proper scrutiny because those plant groups do not attract the attention of anybody else. Errors, inconsistencies and absurdities remain undetected. The professional also goes to the outer limits of his intellect where he/she is just as error‑prone as any other person operating at their extreme. Both classes of enthusiasts ‑‑ the professional and the layman ‑‑ need to draw on some other wisdom to know (probably) what is right and what is wrong.
One of the particular problems faced by both professional botanists and laymen in a popular group of plants, is the profusion of material that comes to be passed around in the horticultural trade without any information on its origin and frequently under the wrong name. This considerably confuses the picture and this confusion is difficult to dispel without reference to populations in the field.
A further problem, again experienced by anyone who does not have extensive experience in the field (and indeed of pattern recognition generally, and not only in living systems), is the quite extraordinary variability of taxa like Haworthia. The degree of variation is not consistent for species or for populations. In extreme cases, where vegetative propagation has occurred, there may indeed be virtually no variation (eg H. reinwardtii); and at the other, hardly two clones in a population are identical. There are no quantum steps where categories like sub-species, varieties etc. have consistent and invariable connotation. The more fundamental and philosophical concept of even the species becomes questionable.
Haworthia has been one case where conflicting views have produced a fair amount of difference of opinion and acrimony among authors, and subsequent confusion in the minds of the audience that need the classification to serve their interest. At present there are two classifications available for Haworthia, the one by C.L. Scott (Scott 1985) and the other by M.B. Bayer (Bayer 1982). Neither has proved unassailable and both have shortcomings. Particular shortcomings of both treatments are that they did not address typification, they did not comprehensively cite herbarium specimens and they did not provide credible identification keys.
In Haworthia the classifications are largely artificial because there are no definite morphological discontinuities between the different species recognised. This is why I have said elsewhere that a truly objective botanical classification would probably reduce the numbers of species to about half of that recognised even by myself. For such an objective classification a key could perhaps be provided. A key was provided in the older Handbooks (e.g. Bayer 1982) but my new classification will not provide a key. The reasons for this are very obvious in my handbooks and in most of my writing on the subject since 1971:-
(1) there are really not enough tangible characters on which to build a key.
(2) where two keys (Scott 1985, Bayer 1982) have been provided, I have no knowledge that anyone has been able to prove their worth or make anything out of them.
Out of about five published reviews of the two accounts by Scott and Bayer, three were quite ambivalent: they did not attempt to test the classifications and did not espouse either. Since a reliable and useful key cannot be constructed, I have concluded that photographs and distribution information are the simplest, most reliable and most direct route to obtaining an identification. The herbarium specimens in the three main South African herbaria follow the revised scheme (Bayer, in ms.) but this could be happenstance rather than cognitive intention.
Part of my strategy in the Handbook (Bayer 1982) was to retain species and varietal names even if the indications were that their status may have been weak. There were two reasons for this. Firstly, classification is also a communication process and I tried to match my classification to what I felt was the mood of the day. Secondly I tried to avoid, where possible, dramatic change which may have had to be reversed, and where there was uncertainty of some kind. Thirdly I retained names where I felt they had value in terms of the information portrayed if not as substantial taxonomic elements. Whether or not I succeeded is beside the point because classification is an ongoing event, based on a sample that is known, on how well it is known and unfortunately on personal perceptions too.
The description of Haworthia reddii (Scott 1994) provides me with an opportunity to evaluate the respective hypotheses of Bayer (1982) and Scott (1985). This account should also be a guide to aspiring taxonomists in the group who may be tempted to start at their own levels of knowledge and competence, rather than properly build on historical fact.
The population upon which H. reddii is based is referred to in the New Haworthia Handbook (Bayer 1982, p.30) under H. batesiana, as follows ‑ “… a collection from Klipplaat northwest of Cathcart is clearly comparable. However, the plants there are too robust to be regarded as H. batesiana and it appears that there is a tendency towards H. cymbiformis“.
Scott did not seem to make the connection between this reference and the plants collected by Dr Reddi and himself at the same place which had in the meantime become better known as Waterdown Dam. This is unfortunate because he does mention both H. batesiana and H. cymbiformis as possible relatives of his new species, and it would have been significant if this was an independent and credible observation.
In 1982, H. batesiana was not well known and there were very few pointers to the reality of its existence. Since then there has been another collection from the Valley of Desolation to confirm its existence there, as well as two collections by P.V. Bruyns from the Kamdeboo Mountains and another from the Tandjiesberg. These are both in the greater Graaff‑Reinet area. From observations on these collections (and several others pertaining to H. archeri), it seems batesiana must be incorporated in H. marumiana as suggested in 1982. Furthermore, the concept of that species needs also to be broadened to include H. archeri and relevant collections (Bayer in ms.).
In Bayer (Haworthiad, 1996) I wrote with reference to H. reddii ‑ “At the time I commented on the Waterdown plants there was some doubt about the existence, whereabouts and whatever of H. batesiana. Since that time there have been any number of collections which fairly conclusively support its inclusion in H. marumiana. There is, so far as I know, still nothing to show that marumiana comes far enough east to support speculation of linkage with cymbiformis via reddii. The area NW of Cathcart to Tarkastad has not been fine‑combed by any collector and it probably would better be regarded as an under‑collected region. Furthermore, the distance from Cathcart to Tarkastad is considerably less than Tarkastad to Beaufort West and Prince Albert (at the western known limits of marumiana). There are plants in the upper Kei collected by Peter Bruyns which may strengthen the view that reddii is associated with cymbiformis. In which case it may be sensible to consider it with the var. lepida. My inclination is to put it with marumiana“.
It is quite obvious from Bayer (1982) that at the time I did not want to commit myself to a decision on the collection from Waterdown Dam. I did not regard it as substantial enough as a single population to justify formal description and was fairly sure that it would fit into either of two already described species. One of these was batesiana, which I suspected would prove to fit into marumiana. The other was cymbiformis. At the time the odds were heavily against the latter because it was not known at all from the Kei River valley, and only slightly better for the former. The nearest populations included the missing H. lepida and a collection of my own from near that site and both of these came from the middle reaches of the Fish River which is rather far to the south. However there were two collections from much further to the east in the Transkei to hint at a more extensive distribution for H. cymbiformis.
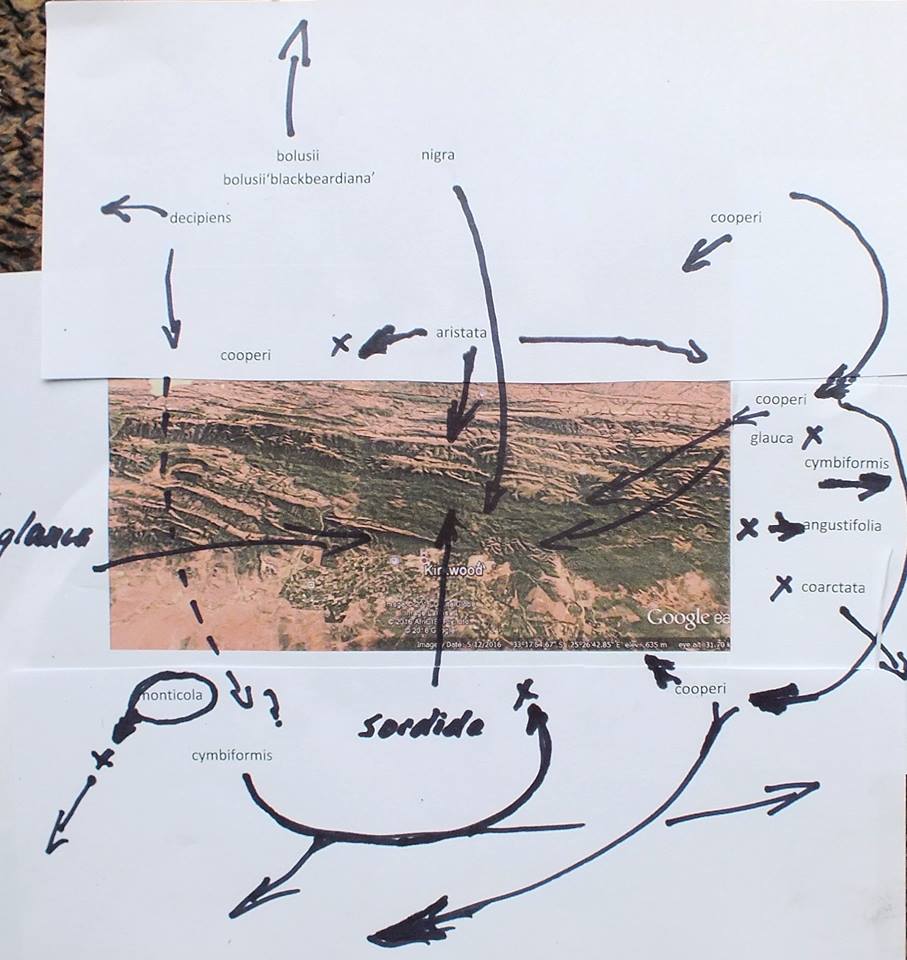
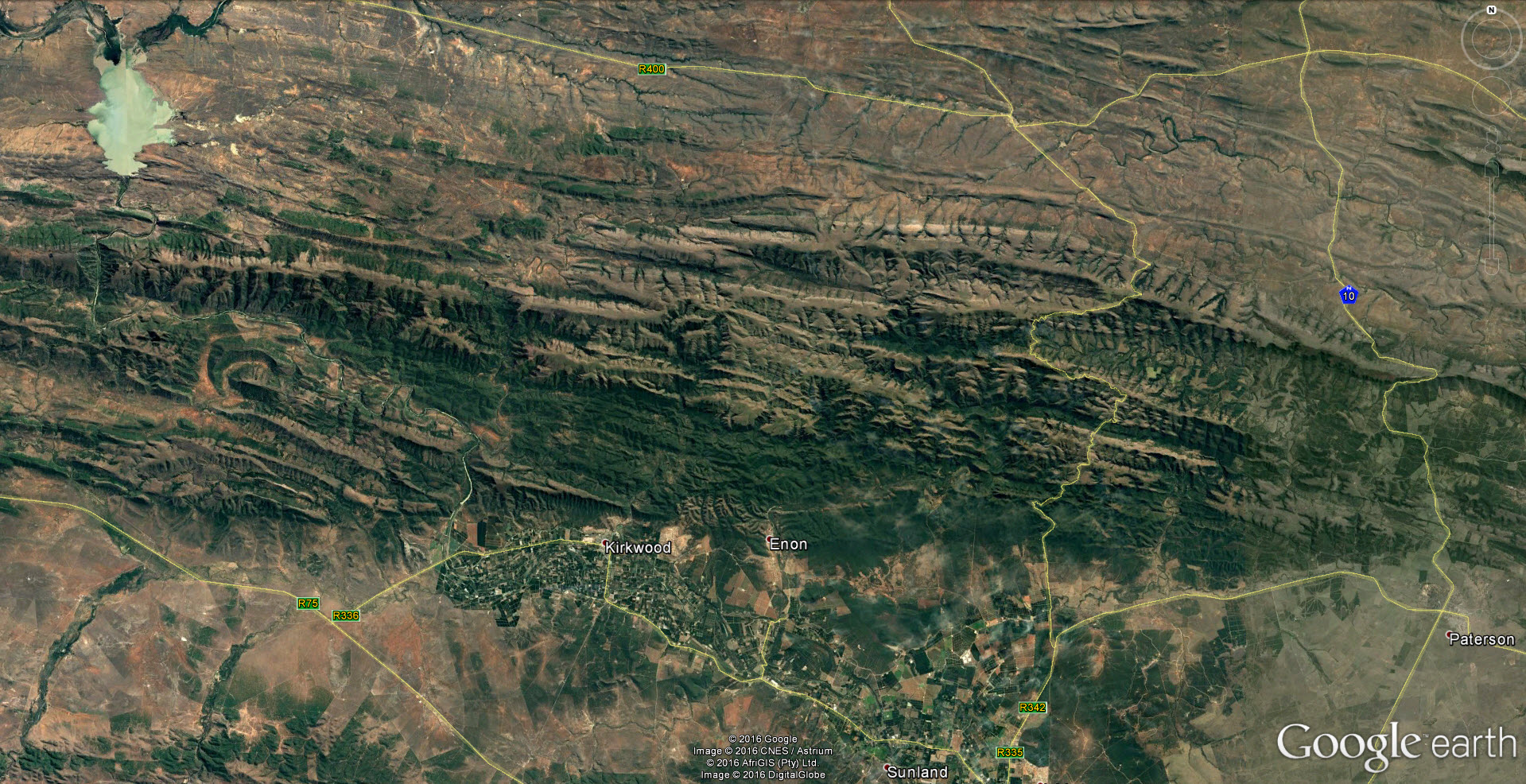
The drainage system of the Kei river and its tributaries is a highly dissected landscape and the terrain is rugged and steep. There are many rocky cliff faces which undoubtedly harbour Haworthias. It will be a very difficult task to thoroughly investigate even a small proportion of possible Haworthia sites. (I did at one time point at the possible significance of river drainage systems regarding species, but it is self‑evident that geographical features of any kind will influence distributions and breeding systems.). Nevertheless, some collections have now been made from which a clearer picture begins to emerge.
The first interesting collections relevant to the ‘reddii‘ problem were collected by P.V. Bruyns at Inverbolo and Inversomo on the Kei River east of Cathcart. These were of H. cymbiformis and established for the first time the existence of this species on the Kei River. He also collected what purports to be H. marumiana var. marumiana in several places north of Queenstown, near to Sterkstroom (in an area which, like Waterdown Dam, is also drained by an upper tributary of the Black Kei). This indicates that H. marumiana also occurs much further east than previously thought. These collections have rather attenuate, strongly spined leaves and are highly marked with translucence between the dense reticulation.
In December 1996, I was fortunate that P.V. Bruyns was able to accompany me on a collecting trip to the Eastern Cape and one of our objectives was the upper Black Kei. It is a tributary of the Black Kei on which the Waterdown Dam was built and the relative location of the populations discussed can be seen on the accompanying map. We were also helped and motivated by a very old specimen in the Pretoria Herbarium collected by Galpin, which I only became aware of earlier in the year and which indicated the occurrence of plants related to H. cymbiformis and ‘reddii‘ southeast of Queenstown.
We travelled on a road running northeast from Cathcart in the direction of the Galpin site, but stopped at the bridge over the Black Kei on the farm Turnstream. Peter did the climbing of the huge south‑facing cliff there and came back with several clones of reddii‑like plants. At the same time I found H. bolusii var. blackbeardiana at the eastern base of the same cliff. We then turned back and travelled eastward along the river to the base of a still higher west‑facing cliff on the farm Highclere. Peter again did the very strenuous climbing and again returned with a few clones which he described as difficult to reach on the vertical cliff face.
Peter’s earlier collection along the lower Kei at Inversomo is still further to the south and east. He also collected H. bolusii var. blackbeardiana at this site.
We took the opportunity to revisit the Waterdown Dam on the way home. I was really surprised to find the south‑facing cliff alongside the dam clothed with huge numbers of plants. Although H. marumiana is also a clump‑former, these larger clumps were at lower altitude and much more accessible than H. marumiana usually is. Some of the plants had very distinctive translucent dots and lines while others are unmarked and uniformly opaque with a faint reticulate patterning on the leaves. The floral characters mentioned by Scott are not definitive although the flowers do appear to have strongly coloured veins. We also noted the presence at Waterdown Dam of H. bolusii var. blackbeardiana. The repeated presence of this species may be important in the context of co‑occurrence which forms the basis of my hypothesis relating geographical distribution to the species concept. It is only slightly relevant to this article but it is critical to a broader understanding of the genus [1].
The offsets we collected from Turnstream, Highclere and Waterdown Dam have taken several months under relatively low light to grow out enough to make useful comment. At the moment it is extremely difficult to see any difference between three distinct clones from Turnstream and the collection made on the same trip from Waterdown Dam. I put it like this because the Waterdown plants are quite variable as to the translucent patterning on the leaves. This may be almost absent, or the margins may be translucent, or the face of the leaves may be quite heavily marked with a series of elongated translucent dots or short lines. (It should be noted that in H. cymbiformis as a whole, there is a vast range of translucent patterning, from virtually absent, to only translucent leaf‑margins, to massive reticulate or dotted translucence). The Turnstream collection comprises a very small sample (smaller than I would have liked, and I would have preferred to have seen the plants in situ if I had been fit enough to do so) but the plants are virtually identical in both shape and size to those Waterdown plants which lack the translucent markings. The colour is also the same rather opaque mid‑green. The leaves are sub‑cylindrical, or flatter and slightly recurved with a faint darker reticulation similar to that in H. marumiana var. batesiana, and which is also often evident in H. cymbiformis. The leaf margins in both the Turnstream and Waterdown collections are relatively smooth with evidence of more spination in a few clones of the bigger Waterdown sample. This spination is not comparable with that of the Andriesberg collections.
The Highclere plants looked slightly different at the time of collection. They were bigger, paler in colour and less opaque. The margins were also more heavily spined. I relate these plants to a wider concept of H. cymbiformis var. setulifera V.Poelln. It seems extremely improbable in the context of Haworthia, that these two populations at Turnstream and Highclere could be different species and I cannot harbour any question of this kind.
The Inverbolo and Inversomo plants have been in cultivation for more than eight years and, as they were also grown under brighter light, a straight comparison is perhaps unwise. In comparison with the Turnstream and Waterdown plants they have relatively short obtuse leaves and form tighter smaller rosettes, the coloration is more intense, slightly more glaucous, and the reticulation, opaqueness and/or translucence in either of the two clones (the sample is too small) representing this collection is practically the same. I included this collection among the specimens of H. cymbiformis var. setulifera, which is indicative of the compounding difficulty of making decisions, already difficult, below species level.
These three collections taken together seem to show a definite and tangible connection between the Waterdown Dam plants on the upper reaches of the Black Kei, through the collection at Turnstream and the herbarium collection of Galpin’s, to H. cymbiformis as represented by the Highclere collection and also the Inverbolo and Inversomo collections further to the south.
The connection to H. marumiana is weaker. As one moves northeastwards from Tarkastad, populations of H. marumiana retain their more plentiful and rather slender leaves and do not tend to become more like the Waterdown collections or like H. cymbiformis. The translucence becomes denser and the plants more spinescent. Collections from the western Karoo (Sutherland, Merweville and Carnarvon by Bruyns and Bayer) which enforce the inclusion of H. archeri under marumiana, weaken the argument to include reddii there too. In particular, forms of H. marumiana var. batesiana which do bear resemblance to the Waterdown Dam collections occur only very far to the west around Graaff-Reinet. The clinal trend in this species from Tarkastad northeastward, is thus rather away from a resemblance to the Waterdown Dam plants than towards it. Thus, if one is to seek continuity of variation, the Waterdown Dam populations do not form part of the series exhibited by H. marumiana but fit into the series of variants now known in H. cymbiformis along the upper reaches of the Kei River.
In 1982 I postulated that there were two species viz. H. batesiana and H. cymbiformis, involved in an assessment of the Waterdown Dam collections and was unable to fit it conclusively into either of these. Nevertheless, I was convinced that it could be accommodated here and this was, and has been, the prediction of my classification hypothesis.
More recent collections (mostly by P.V. Bruyns) have filled in much detail in the distributions of both H. cymbiformis and H. marumiana that was, at that time, unknown. These have indicated that H. batesiana and H. archeri can be included in a broader concept of H. marumiana (also as predicted), and they have amplified the known information on H. cymbiformis. This new information shows that, if the species concepts of geographical continuity and co‑occurrence are followed, ‘reddii‘ is not a discrete new element standing outside of known and variable species. My prediction that it should not be accommodated in H. batesiana (i.e. H. marumiana) seems to be correct, and our investigations seem to confirm rather that it is an integral part of H. cymbiformis. The important fact then is that recognition as a distinct species is not warranted, and it can adequately be discussed and classified in terms of the structure of the Haworthia Handbooks. The hypothesis has not been disproved and there is a rational basis for development of a still better understanding.
An important implication has been that if reddii and similar individual populations are to be treated as distinct species, then each new discovery of which there could be many, will require a new name and the system will become increasingly disordered and fail. Evidence of exactly this problem is presented by the descriptions of H. batteniae, H. pringlei, H. joeyae, H. venusta and H. mcmurtryi all of which can similarly be accommodated within other variable species. As far as variability is concerned, the plants from Waterdown Dam, and other populations now associated with them, are not exceptional. If we had to continue naming each apparently different element like this, we would end up with a structure that has no coherence, no predictive element and no value in the sense that botanical classification is required to express ‘pattern’ and carry information generally. Such a system may work for the collector in that he may have a name for a particular clone or set of clones, but have no wider or deeper meaning. Many people may be comfortable with and feel justified in using Scott’s treatment. Nevertheless, such an approach simply does not accommodate the incredible variation within the genus.
Because of the conflicting views that seem to be an inevitable part of the process of plant classification, many commentators have said that it is not a science but an art. However, this conflict should not be there. The essence of science is replication i.e. the deduction of conclusions (for example, a classification) from experiments (for example, observations on plants) which should be repeatable. In my work on Haworthia, I have been very conscious of the historic conflict in the genus, the need for credibility, and the responsibility attached to making public statements. Unfortunately, while I may have made mistakes, other authors seem to be less conscientious. Therefore the presentation of differing taxonomic treatments requires the reader to discriminate between them. This demands of the reader that he consider carefully the evidence put forward by authors and then discriminate for himself which author has concluded correctly. Most readers are not prepared to go to this amount of trouble and would rather declare the taxonomy of the group concerned to be ‘controversial’. This is unfair to all authors and also to other readers as it discounts the effort and sacrifice these people put into collecting and communicating information. The opinion that taxonomy is an art with little relevance to the enjoyment of the plants themselves, stems from intellectual laziness and ignorance. It belies the fact that the mere conveyance of a name, which can be forgotten in the very same moment, satisfies some deep psychological need. The audience also has the responsibility to think analytically and critically about what is laid before them. Otherwise they may get a meaningless classification that they have earned, but which is just another yoke around the neck of others who may be striving for the light.
[1] My classification hypothesis is built on a definition of species which pre-supposes that they are ‘continuous genetically and morphologically in space and/or time’ (Bayer 1982). Therefore the prediction then is that not only is it probable that H. cymbiformis and H. marumiana will eventually found to be continuous in geographic space, but it is probable that they will also be found to be continuous with H. bolusii. The entire hypothesis should fit within the framework of taxonomic botany, whatever the level of expertise, and satisfy the requirements of scientific discipline. Any two people should come to the same conclusion. If there is conflict there is error.













![H. cymbiformis - Curtis’s Botanical Magazine, vol. 21 t. 802 (1805) [S.T. Edwards]8067](https://haworthia-updates.haworthia.org/wp-content/uploads/1999/01/H.-cymbiformis-Curtis’s-Botanical-Magazine-vol.-21-t.-802-1805-S.T.-Edwards8067-181x300.jpg)
![H. cuspida - Addisonia, vol. 23 t. 741 (1954-1959) [M.E. Eaton] 162903](https://haworthia-updates.haworthia.org/wp-content/uploads/1999/01/H.-cuspida-Addisonia-vol.-23-t.-741-1954-1959-M.E.-Eaton-162903-191x300.jpg)